Electronegativity - Definition, Examples, Quiz, FAQ, Trivia
Discover how atoms attract electrons in chemical bonds
What is Electronegativity?
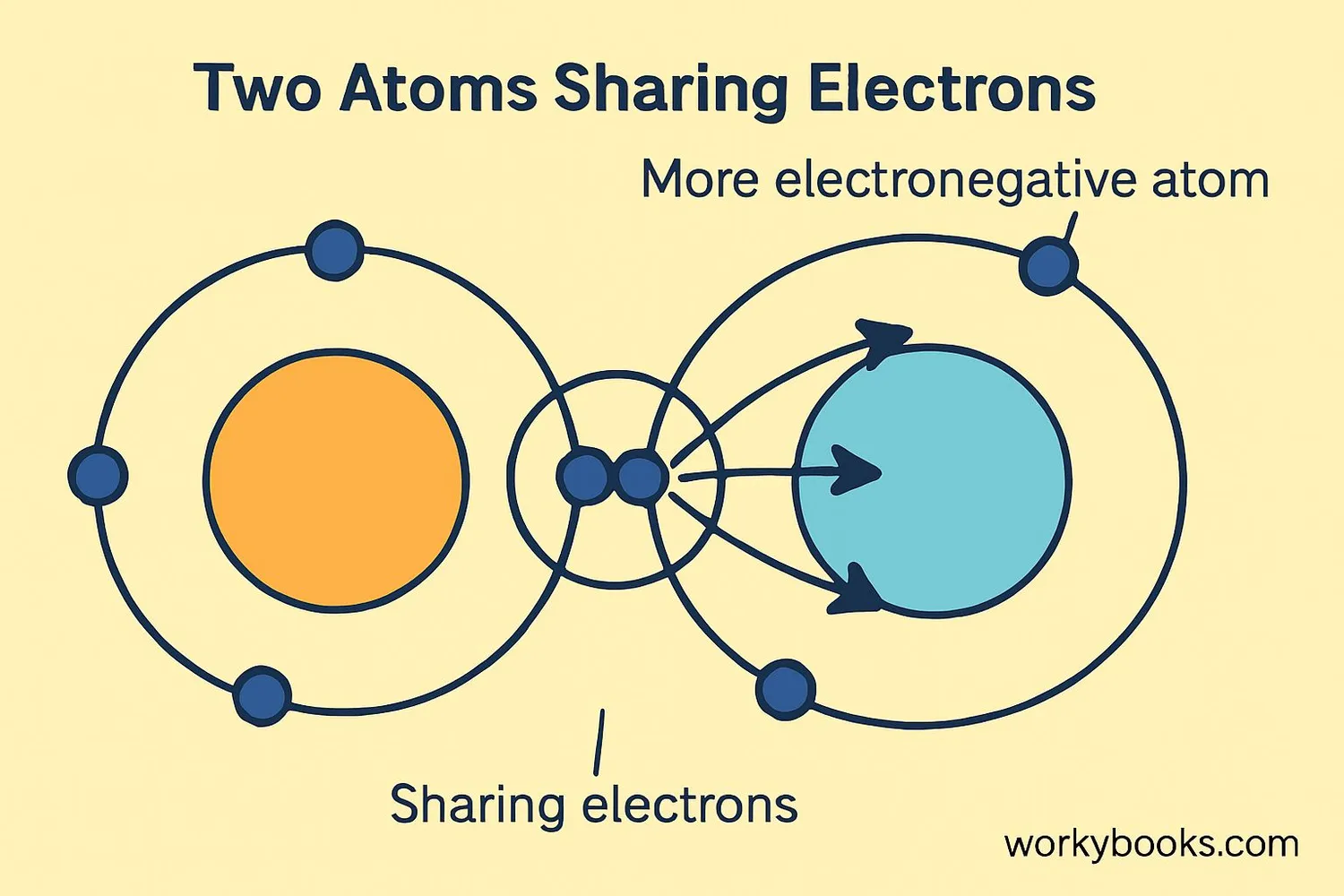
Electronegativity is a measure of how strongly an atom attracts electrons when it forms a chemical bond. Think of it as an atom's "electron-pulling power"!
Atoms with high electronegativity pull electrons toward themselves more strongly. Fluorine is the most electronegative element, while cesium and francium have the lowest electronegativity values.
This concept helps us understand why some bonds are stronger than others and why molecules have specific shapes.
Science Fact!
Electronegativity was first described by Linus Pauling in 1932, who created the Pauling scale that we still use today!
Periodic Trends in Electronegativity
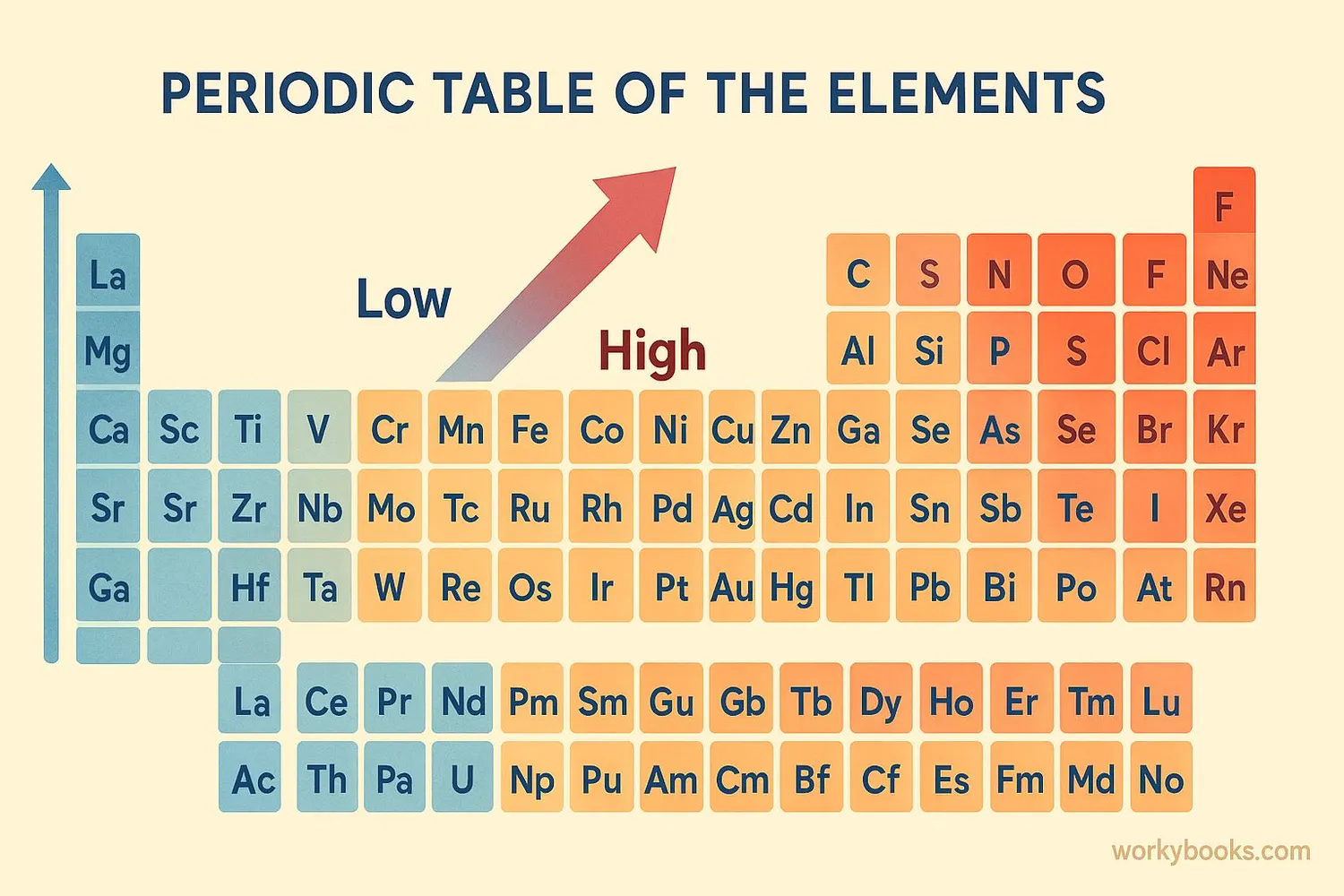
Electronegativity follows predictable patterns on the periodic table:
Across a Period
Electronegativity increases from left to right
Down a Group
Electronegativity decreases from top to bottom
Highest Values
Top right corner (fluorine has highest)
Lowest Values
Bottom left corner (francium has lowest)
The Pauling scale is the most common way to measure electronegativity. Fluorine is assigned a value of 4.0, the highest possible, while cesium and francium have values around 0.7.
Why These Trends?
As you move right across a period, atoms have more protons but the same electron shells, so they pull electrons more strongly. Moving down a group, atoms have more electron shells that shield the nucleus' pull.
Electronegativity in Chemical Bonding

Electronegativity helps us predict what type of bond will form between atoms:
Nonpolar Covalent
Electronegativity difference < 0.4
Equal sharing of electrons
Polar Covalent
Difference 0.4 to 1.7
Unequal sharing creates partial charges
Ionic Bond
Difference > 1.7
Electrons transferred completely
For example:
• H₂ (hydrogen gas) has identical atoms - nonpolar covalent bond
• H₂O (water) has oxygen more electronegative - polar covalent bond
• NaCl (salt) has large difference - ionic bond
This also explains why water molecules form hydrogen bonds and why salt dissolves in water!
Electronegativity Quiz
Test your understanding with this electronegativity quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about electronegativity:
Electronegativity Trivia
Discover fascinating facts about electronegativity:
The Electron King
Fluorine is so electronegative that it can form compounds with noble gases like xenon and krypton, which were once thought to be completely unreactive!
Double Nobel Winner
Linus Pauling, who created the electronegativity scale, is one of only four people to win two Nobel Prizes (Chemistry in 1954 and Peace in 1962).
Water's Special Properties
Water's bent shape and amazing properties come from oxygen's high electronegativity, which creates partial positive and negative charges on the molecule.
Multiple Scales
Besides the Pauling scale, scientists also use the Mulliken and Allred-Rochow scales to measure electronegativity, each with slightly different values.




