Chemical Symbols - Definition, Examples, Quiz, FAQ, Trivia
Discover the language of chemistry through symbols, formulas, and the periodic table!
Chemical Symbols
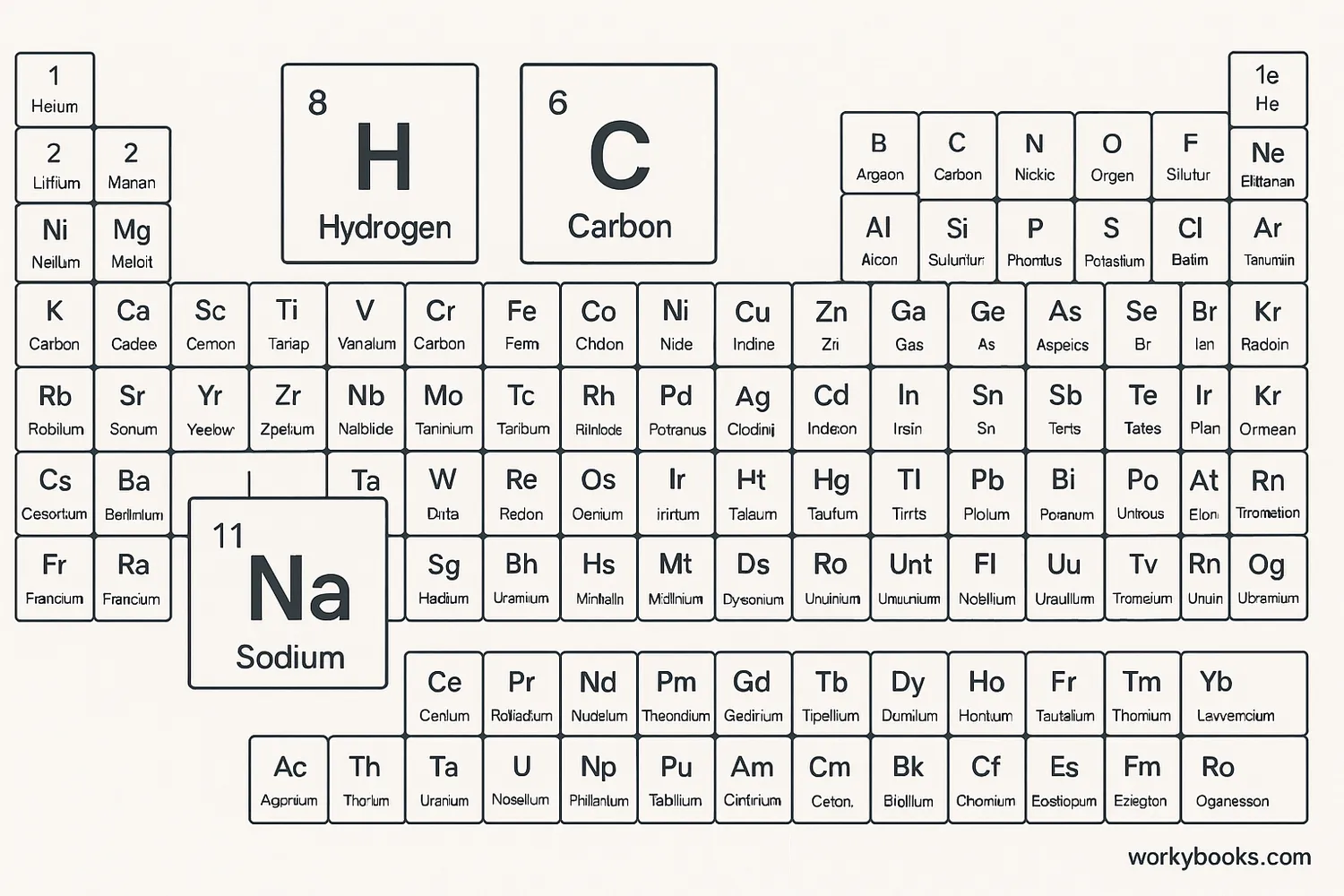
Chemical symbols are the abbreviations used in chemistry for chemical elements. Each element has a unique one or two-letter symbol that makes it easier to write chemical formulas and equations.
For example, the symbol for hydrogen is H, oxygen is O, and carbon is C. Some symbols come from their Latin names, like sodium which is Na from the Latin word "natrium".
Science Fact!
The system of chemical symbols was developed by Swedish chemist Jöns Jacob Berzelius in 1813. His system is the basis for the symbols we use today.
The Periodic Table
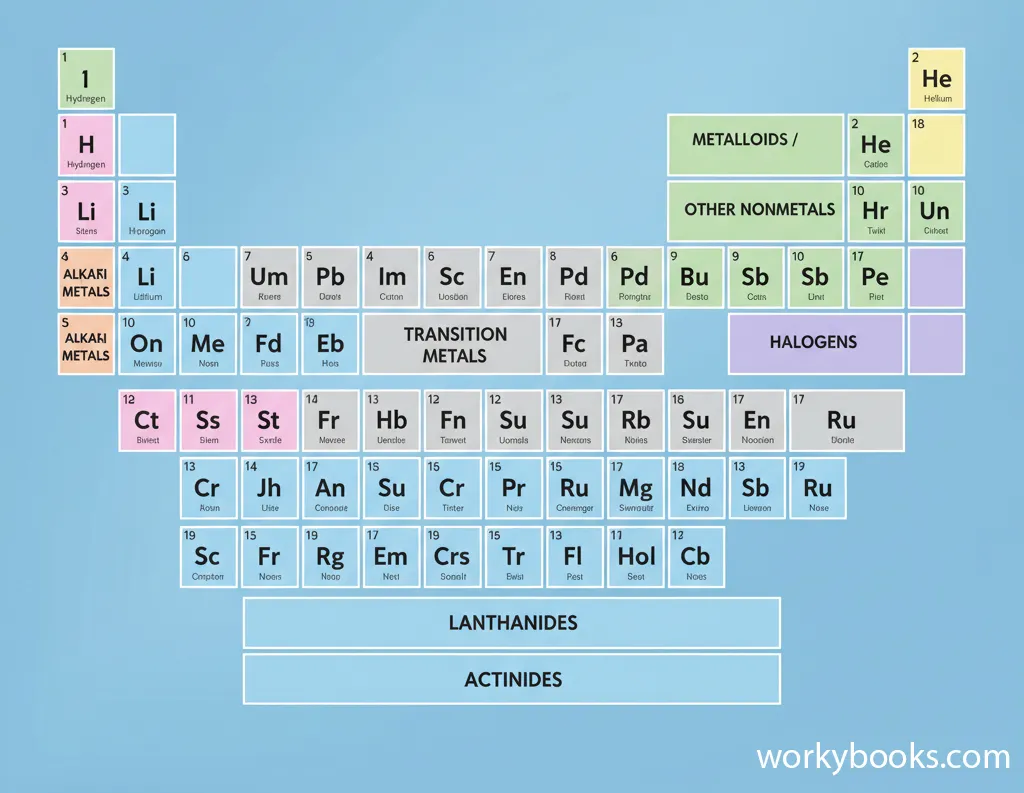
The periodic table is a chart that organizes all known chemical elements based on their properties. Elements are arranged in order of increasing atomic number (the number of protons in their atoms).
The table has rows called periods and columns called groups. Elements in the same group often have similar chemical properties. For example, all elements in Group 1 (the first column) are very reactive metals.
Atomic Number
The number of protons in an atom's nucleus
Atomic Symbol
The one or two-letter abbreviation for the element
Atomic Weight
The average mass of atoms of that element
Element Name
The full name of the chemical element
The periodic table helps scientists predict how elements will react with each other. It's one of the most important tools in chemistry!
Chemical Formulas
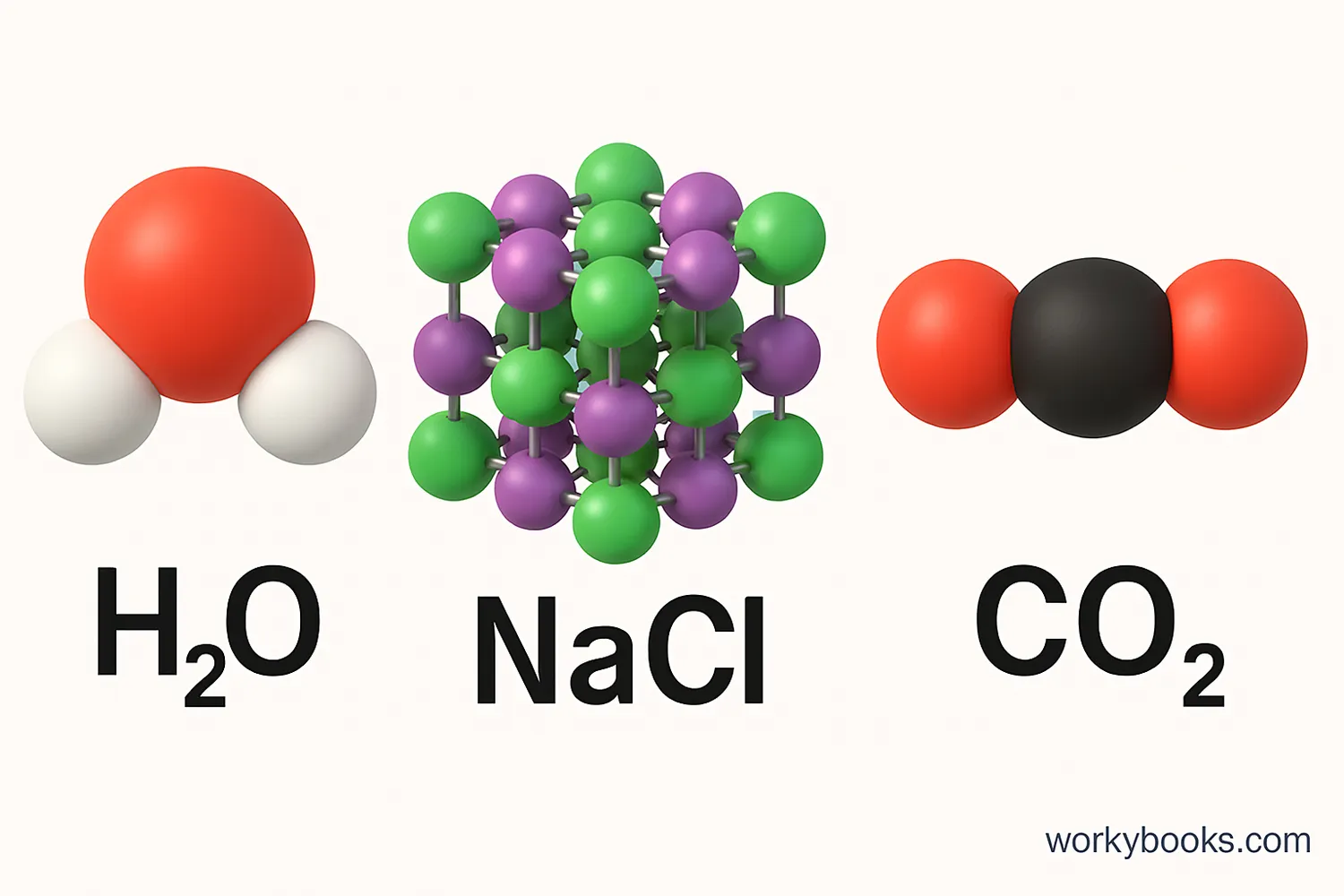
Chemical formulas are combinations of chemical symbols that represent compounds. They show which elements are in the compound and in what ratio.
For example, the formula for water is H2O, which means each water molecule has 2 hydrogen atoms and 1 oxygen atom. The formula for table salt is NaCl, which means it contains sodium and chlorine in a 1:1 ratio.
Subscripts
Numbers written slightly below the line (like the 2 in H2O) show how many atoms of that element are in one molecule of the compound.
Coefficients
Numbers in front of formulas (like the 2 in 2H2O) show how many molecules are involved in a reaction.
Chemical formulas help scientists communicate precisely about substances without having to write out long names.
Atoms and Elements
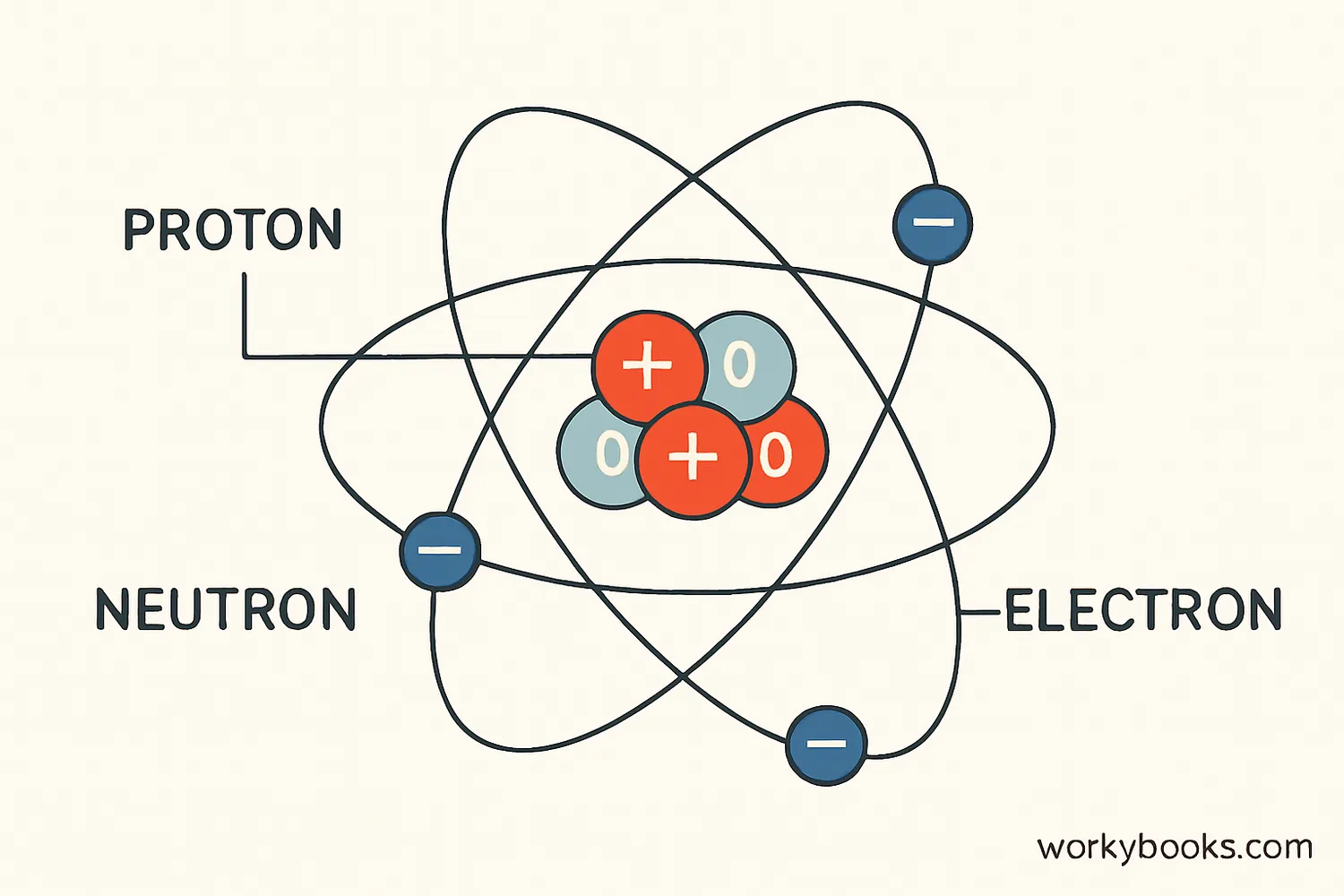
Atoms are the basic building blocks of all matter. They're incredibly small—millions could fit on the head of a pin! Each atom consists of a nucleus containing protons and neutrons, with electrons orbiting around it.
An element is a substance made of only one type of atom. For example, a piece of pure gold contains only gold atoms, and a tank of helium gas contains only helium atoms.
Protons
Positively charged particles in the nucleus that determine the element's identity
Neutrons
Neutral particles in the nucleus that add mass to the atom
Electrons
Negatively charged particles that orbit the nucleus and participate in chemical bonding
Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom. These electrons determine how an element will react with other elements. Elements with the same number of valence electrons often have similar chemical properties.
Molecules and Compounds
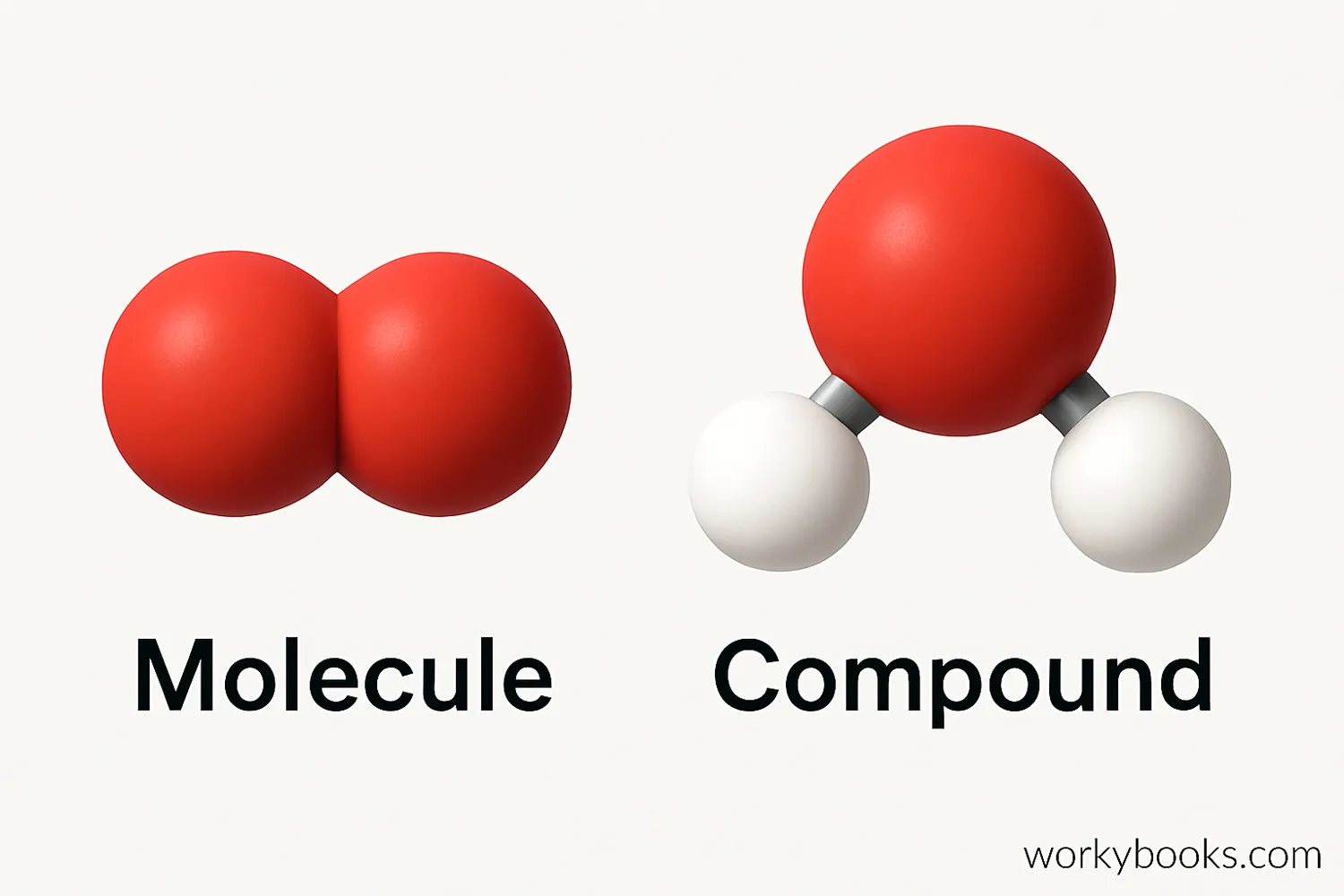
When atoms bond together, they form molecules. A molecule can be made of the same type of atoms (like O2, oxygen gas) or different types of atoms (like H2O, water).
A compound is a substance made of two or more different elements that are chemically bonded together in fixed proportions. Water (H2O) and table salt (NaCl) are both compounds.
Organic Chemistry
The study of carbon-containing compounds, which include most chemicals found in living things like proteins, carbohydrates, and DNA.
Inorganic Chemistry
The study of compounds that don't contain carbon (with a few exceptions), including minerals, metals, and salts.
Chemical equations show how substances change during chemical reactions. They have reactants (starting materials) on the left, an arrow, and products (ending materials) on the right.
This equation shows that two molecules of hydrogen gas react with one molecule of oxygen gas to form two molecules of water.
Chemistry Quiz
Test your understanding of chemical symbols and formulas with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about chemical symbols and formulas:
Science Trivia
Discover some amazing facts about chemical symbols and the periodic table!
Element Origins
Elements get their names from various sources: some from scientists (Einsteinium, Curium), some from places (Americium, Francium), some from mythology (Thorium, Mercury), and some from properties (Chlorine from the Greek "chloros" meaning greenish-yellow).
Missing Elements
When Dmitri Mendeleev created the first periodic table in 1869, he left gaps for elements that hadn't been discovered yet. He predicted the properties of these missing elements with remarkable accuracy, and within 15 years, three of them (gallium, scandium, and germanium) were discovered.
Most Abundant Elements
The most abundant element in the universe is hydrogen, which makes up about 75% of all matter. On Earth, the most abundant element in the crust is oxygen (about 47%), followed by silicon (28%), and aluminum (8%).
Unique Element States
Most elements are solids at room temperature, but 11 are gases and only 2 are liquids: mercury and bromine. Some elements can even change state dramatically - tungsten has the highest melting point (3422°C) while helium has the lowest boiling point (-269°C).





