What Are Polymers - Definition, Examples, Quiz, FAQ, Trivia
Discover the amazing molecules that make up our world!
What is a Polymer?
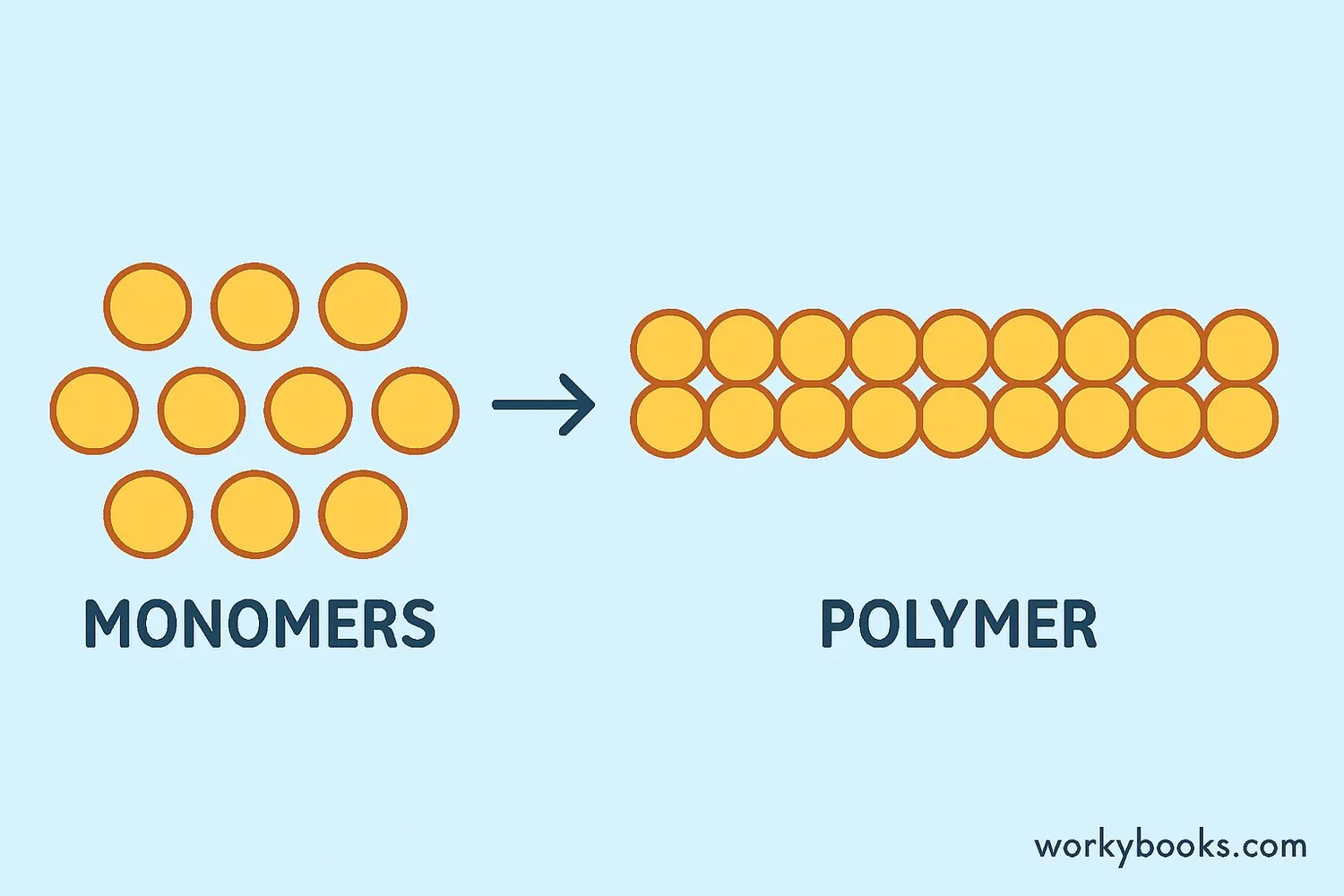
A polymer is a large molecule made up of many smaller repeating units called monomers. The word "polymer" comes from Greek words meaning "many parts". Polymers are all around us - in plastics, rubber, fabrics, and even in our own bodies!
Think of polymers like a train: each train car is a monomer, and the whole train is the polymer. When many small molecules (monomers) join together through a process called polymerization, they form long chains that create materials with special properties.
Polymer Fact!
DNA in your body is a natural polymer made of repeating units called nucleotides. This polymer carries your genetic information!
Types of Polymers
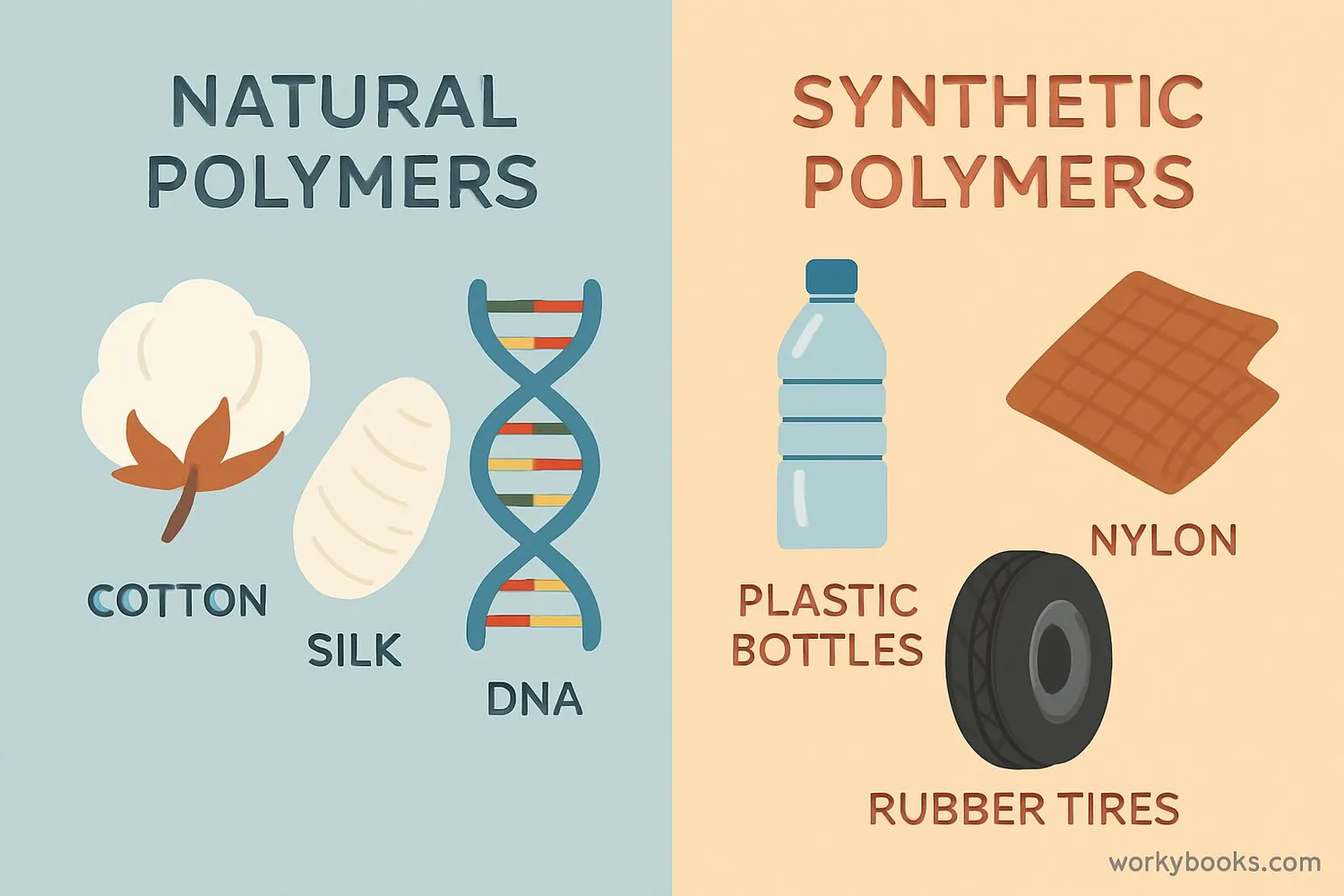
Polymers can be divided into two main categories: natural and synthetic. Both types play important roles in our world!
Natural Polymers
Created by nature: DNA, proteins, cellulose (in plants), natural rubber, silk, wool
Synthetic Polymers
Created by humans: plastics (polyethylene, PVC), nylon, polyester, Teflon, silicone
Synthetic polymers can be further categorized:
• Thermoplastics (can be melted and reshaped)
• Thermosetting polymers (set permanently when formed)
• Elastomers (stretchy materials like rubber)
Polymers can also be classified as homopolymers (made of one monomer type) or copolymers (made of two or more monomer types).
Did You Know?
Cellulose, the polymer that makes up plant cell walls, is the most abundant natural polymer on Earth!
Polymerization Process
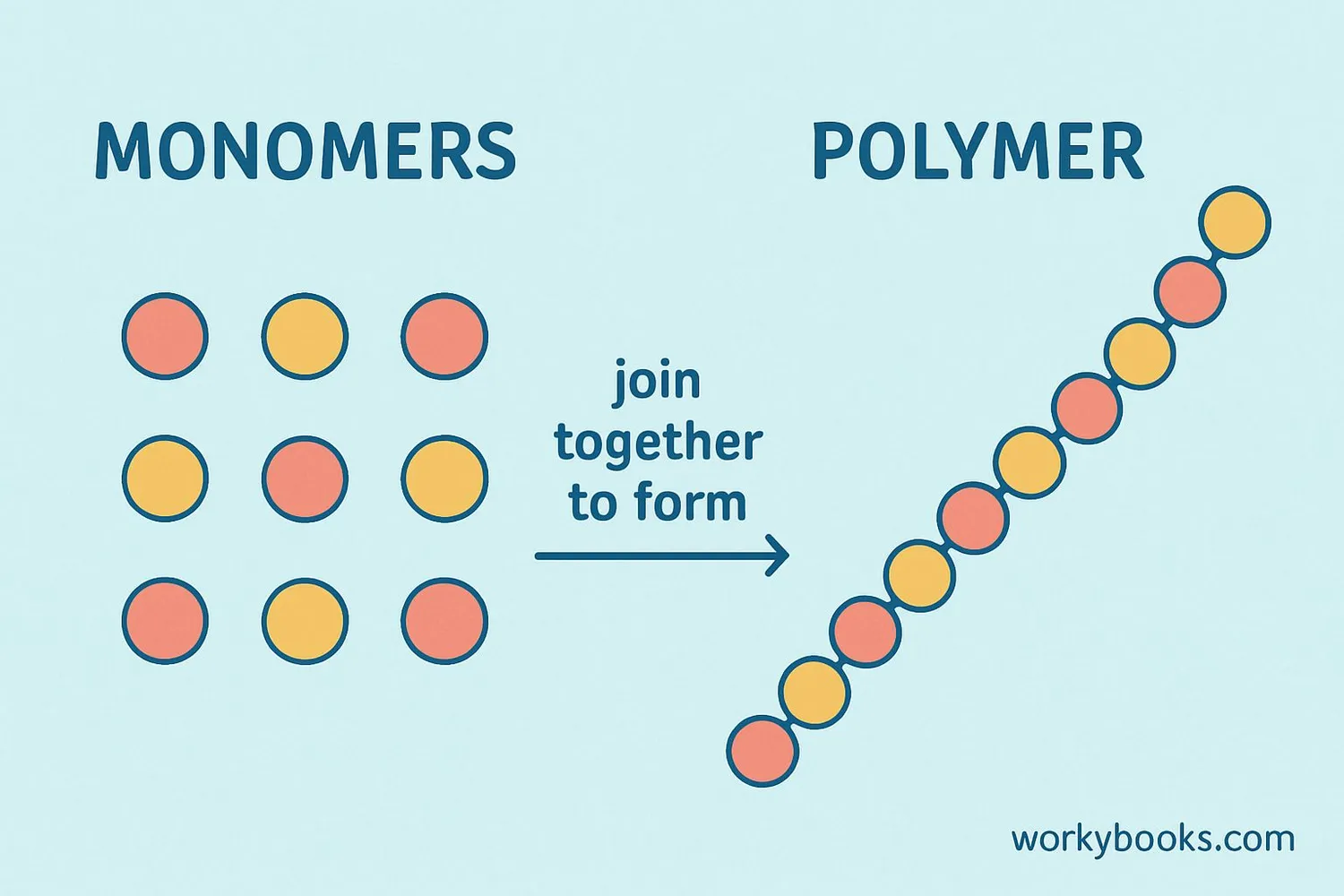
Polymerization is the chemical process where monomers join together to form polymers. There are two main types of polymerization:
Addition Polymerization
Monomers add to a growing chain without losing any atoms. Used for plastics like polyethylene and PVC.
Condensation Polymerization
Monomers join with the loss of small molecules like water. Used for nylon and polyester.
The structure of a polymer affects its properties. Linear polymers form straight chains, while branched polymers have side chains. Cross-linked polymers have connections between chains, making them stronger.
Important polymer properties include:
• Tensile strength (resistance to pulling)
• Elasticity (ability to stretch)
• Glass transition temperature (when it becomes brittle)
• Crystallinity (how orderly the molecules are)
Polymer Applications
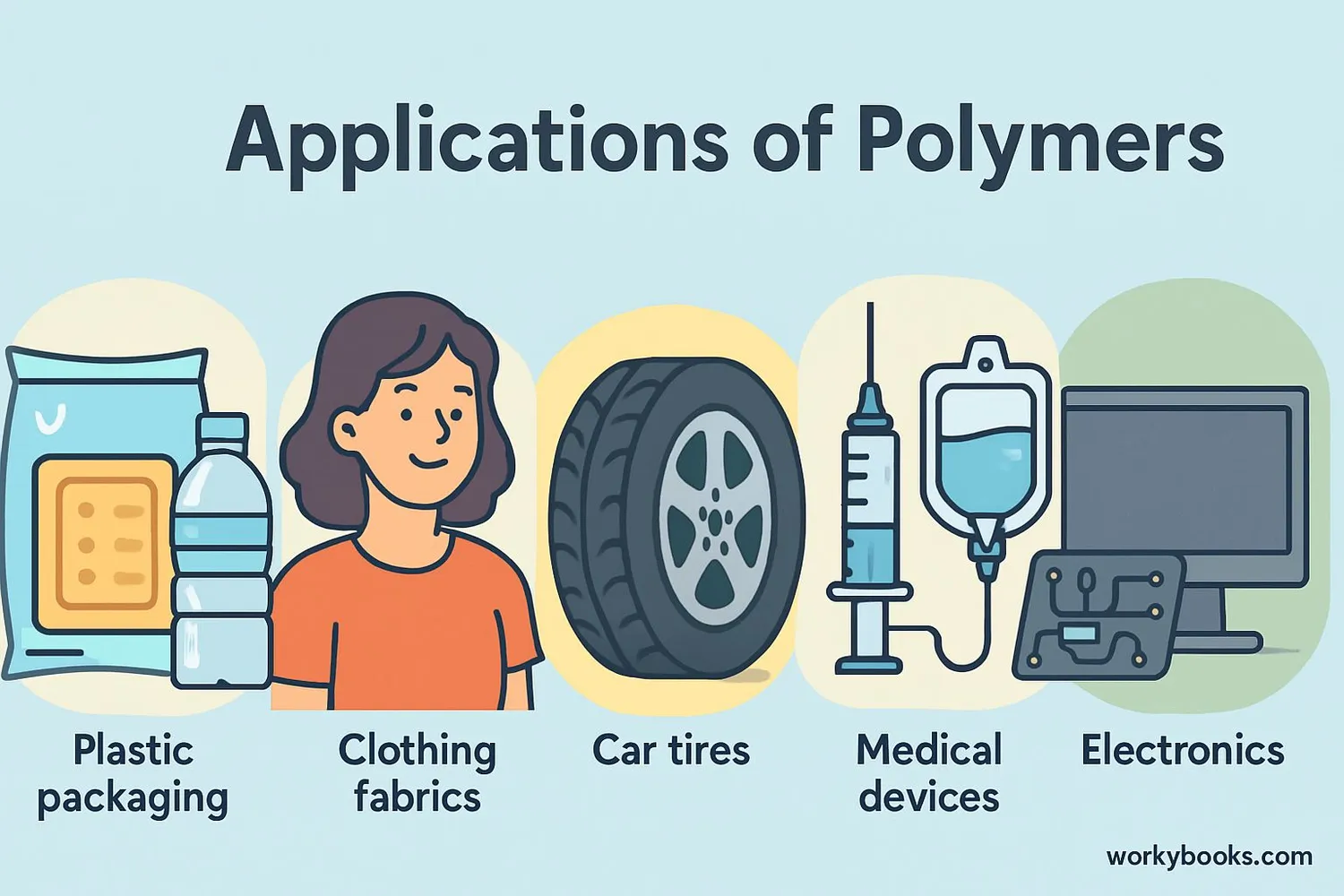
Polymers are incredibly versatile materials that we use in almost every aspect of modern life. Their unique properties make them suitable for countless applications:
Clothing & Textiles
Polyester, nylon, acrylic, and spandex are all synthetic polymers used in clothing.
Automotive
Tires, dashboards, bumpers, seat covers, and insulation materials.
Medical
Surgical gloves, syringes, artificial joints, and drug delivery systems.
Other important polymer applications include:
• Packaging: Plastic containers, bottles, and food wraps
• Electronics: Insulating materials for wires and circuit boards
• Construction: Pipes, insulation, paints, and adhesives
• Sports Equipment: Helmets, balls, and protective gear
Biomedical polymers are particularly important for drug delivery systems and tissue engineering, helping doctors treat diseases and repair injuries.
Polymer Quiz
Test your polymer knowledge with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about polymers:
Fun Polymer Trivia
Discover some amazing facts about polymers!
Ancient Polymers
Natural polymers have been used for thousands of years! Ancient Mesoamericans used natural rubber from rubber trees around 1600 BC to make balls for games.
The Largest Polymer
The largest natural polymer in your body is DNA! If you unraveled all the DNA in one human cell, it would stretch about 2 meters long. All the DNA in your body would stretch to the sun and back over 300 times!
Recycling Challenge
Less than 10% of all plastic ever made has been recycled. Scientists are working on creating new biodegradable polymers and better recycling methods to solve this challenge.
Polymer Innovation
Scientists have created polymers that can repair themselves when damaged! These materials have tiny capsules that break open to release a healing agent when cracks form.





