Radioactive Decay - Definition, Examples, Quiz, FAQ, Trivia
Discover how unstable atoms transform and release energy over time
What is Radioactive Decay?
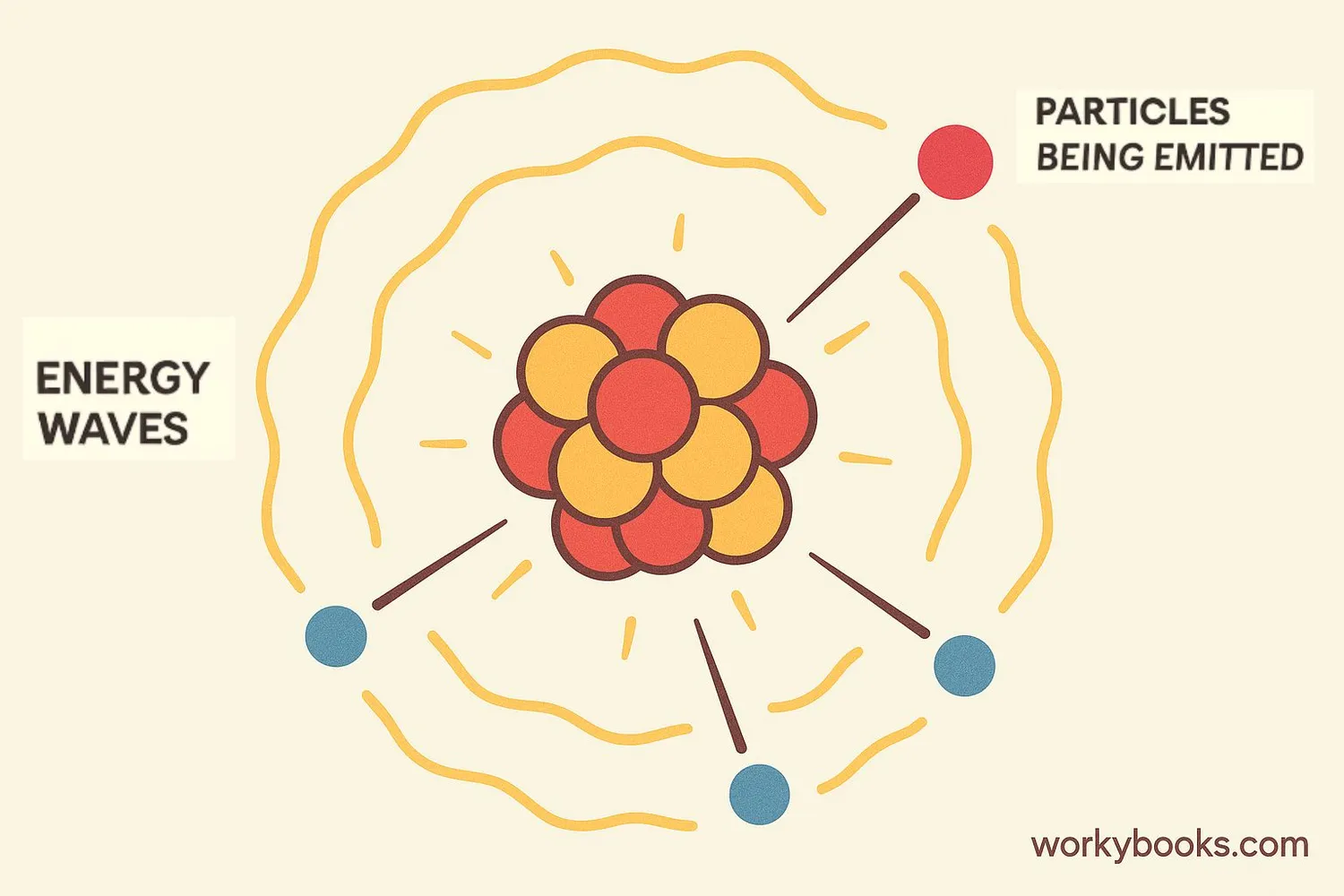
Radioactive decay is a natural process where unstable atoms transform into more stable forms by releasing particles and energy. Think of it like a wobbly table that eventually settles into a stable position—except with atoms, this process releases energy we call radiation.
All matter is made of atoms, and some atoms have unstable nuclei (the center part). To become stable, these atoms emit particles and energy. This process happens randomly but predictably for large groups of atoms. Radioactive decay occurs naturally in elements like uranium, radium, and even in some forms of carbon.
Did You Know?
Your body contains naturally radioactive elements like potassium-40! You're actually slightly radioactive, but at completely safe levels.
Types of Radioactive Decay
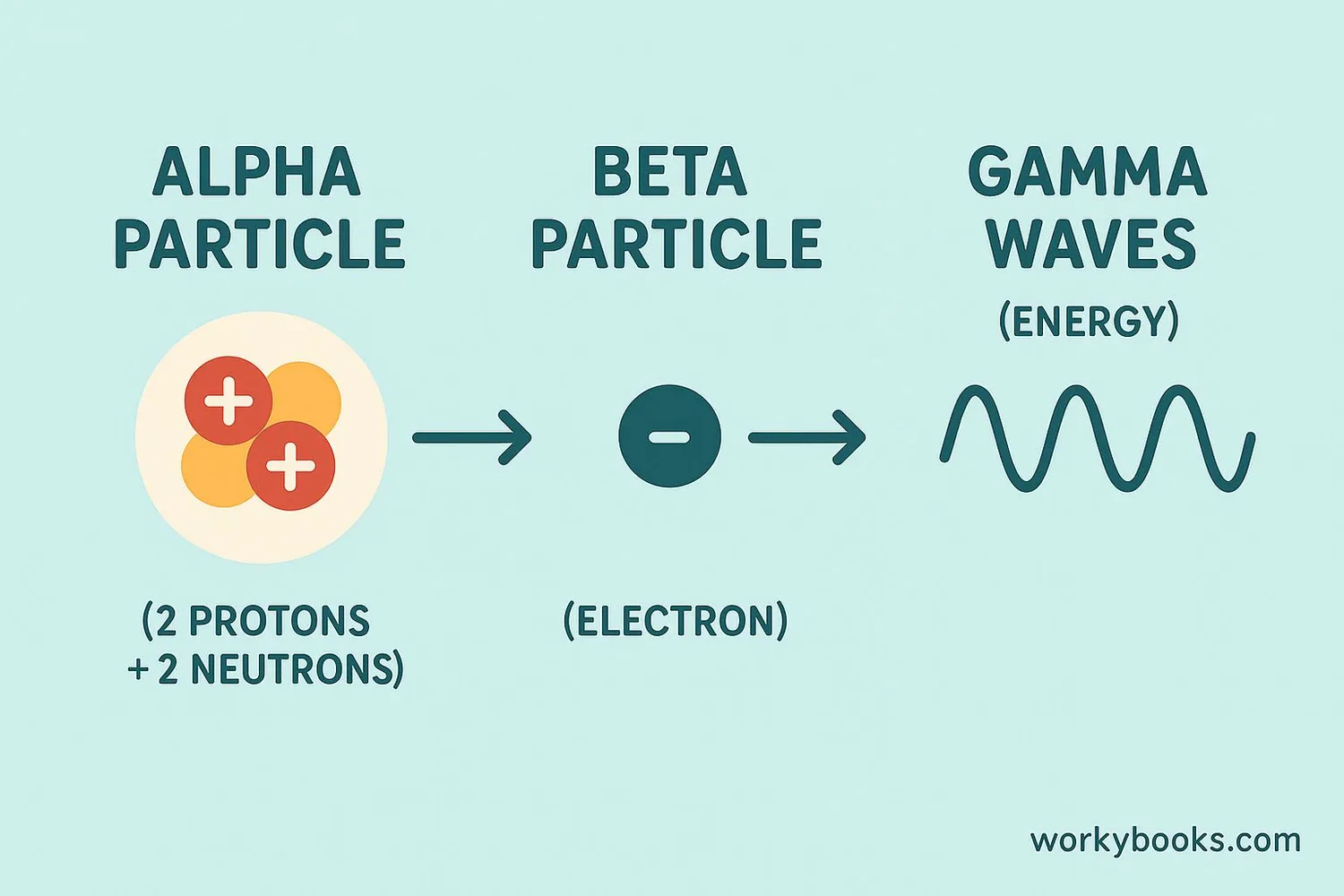
There are three main types of radioactive decay. Each type involves different particles being released from the unstable atom:
Alpha Decay
An alpha particle (2 protons + 2 neutrons) is released. This is like the atom "spitting out" a small piece of itself. Alpha particles can be stopped by paper or skin.
Beta Decay
A beta particle (electron) is released when a neutron turns into a proton. Beta particles can penetrate further than alpha but can be stopped by aluminum.
Gamma Decay
Pure energy (gamma rays) is released without changing the particle composition. Gamma rays are high-energy waves that require thick lead or concrete to stop.
Each type of decay changes the original atom into a different element. For example, when uranium undergoes alpha decay, it becomes thorium. This process continues through a "decay chain" until a stable element (like lead) is formed.
Understanding Half-Life
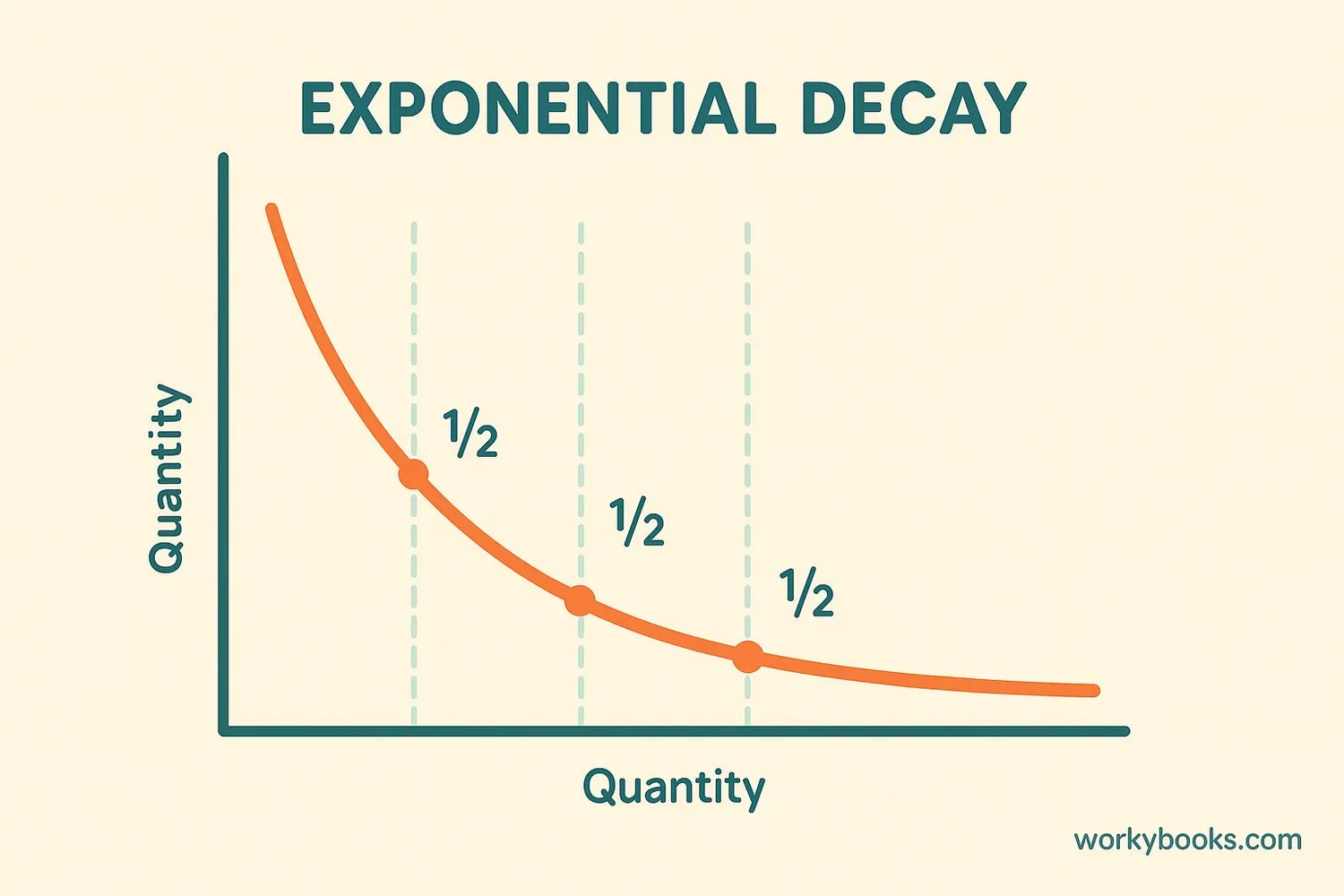
The half-life of a radioactive element is the time it takes for half of the atoms in a sample to decay. It's like a radioactive clock that ticks at a predictable rate!
Different elements have different half-lives:
Short Half-Lives
Some elements decay quickly. For example, radon-222 has a half-life of just 3.8 days.
Medium Half-Lives
Carbon-14, used in dating ancient objects, has a half-life of 5,730 years.
Long Half-Lives
Uranium-238 has a half-life of 4.5 billion years—about the age of Earth!
Scientists use half-life measurements for many applications, including determining the age of fossils and archaeological artifacts (carbon dating), medical treatments, and understanding Earth's geological history.
Real-World Application
Doctors use radioactive elements with short half-lives for medical imaging. These elements decay quickly, minimizing radiation exposure to patients.
Radioactive Decay Knowledge Check
Test your understanding of radioactive decay with these questions. Select the best answer for each question.
Frequently Asked Questions
Here are answers to common questions about radioactive decay:
Interesting Facts About Radioactive Decay
Discover some fascinating information about radioactive decay:
Banana Equivalent Dose
Bananas are naturally radioactive due to their potassium content! Eating one banana exposes you to about 0.1 microsieverts of radiation—a unit scientists call "Banana Equivalent Dose."
Ancient Time Capsules
Radioactive decay allows us to date objects from the distant past. The oldest rocks on Earth (about 4 billion years old) and moon rocks were dated using uranium-lead decay methods.
Earth's Internal Heat
Radioactive decay in Earth's core provides about half of our planet's internal heat! This heat drives volcanic activity and plate tectonics that shape Earth's surface.
Nuclear Spacecraft
Space missions to distant planets often use radioisotope thermoelectric generators (RTGs) that harness heat from radioactive decay to power spacecraft where solar energy is too weak.





