Anions - Definition, Examples, Quiz, FAQ, Trivia
Discover the world of negative ions and their role in chemistry
What is an Anion?
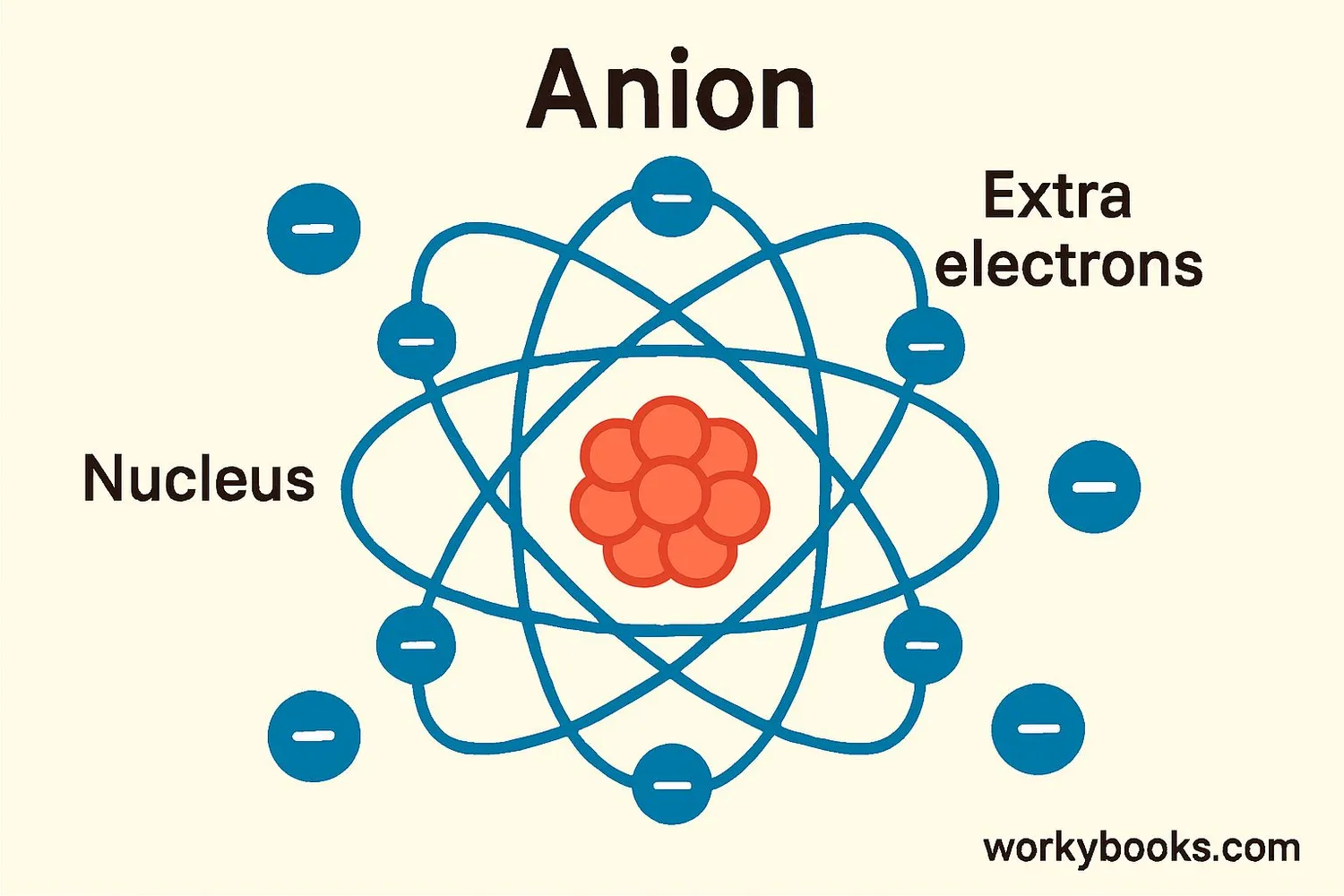
An anion (pronounced AN-eye-on) is a negatively charged ion. But what does that mean? Let's break it down:
• Ions are atoms or molecules that have gained or lost electrons
• Anions have gained one or more extra electrons
• This extra negative charge makes them attracted to positive ions
Think of anions as atoms that have gained extra "negative power" by collecting additional electrons. They're like magnets with a negative pole!
Key Fact!
Anions are formed when atoms gain electrons. The name "anion" comes from the Greek word "ano," meaning "up," because anions move toward the positive electrode (anode) during electrolysis.
How Anions Form
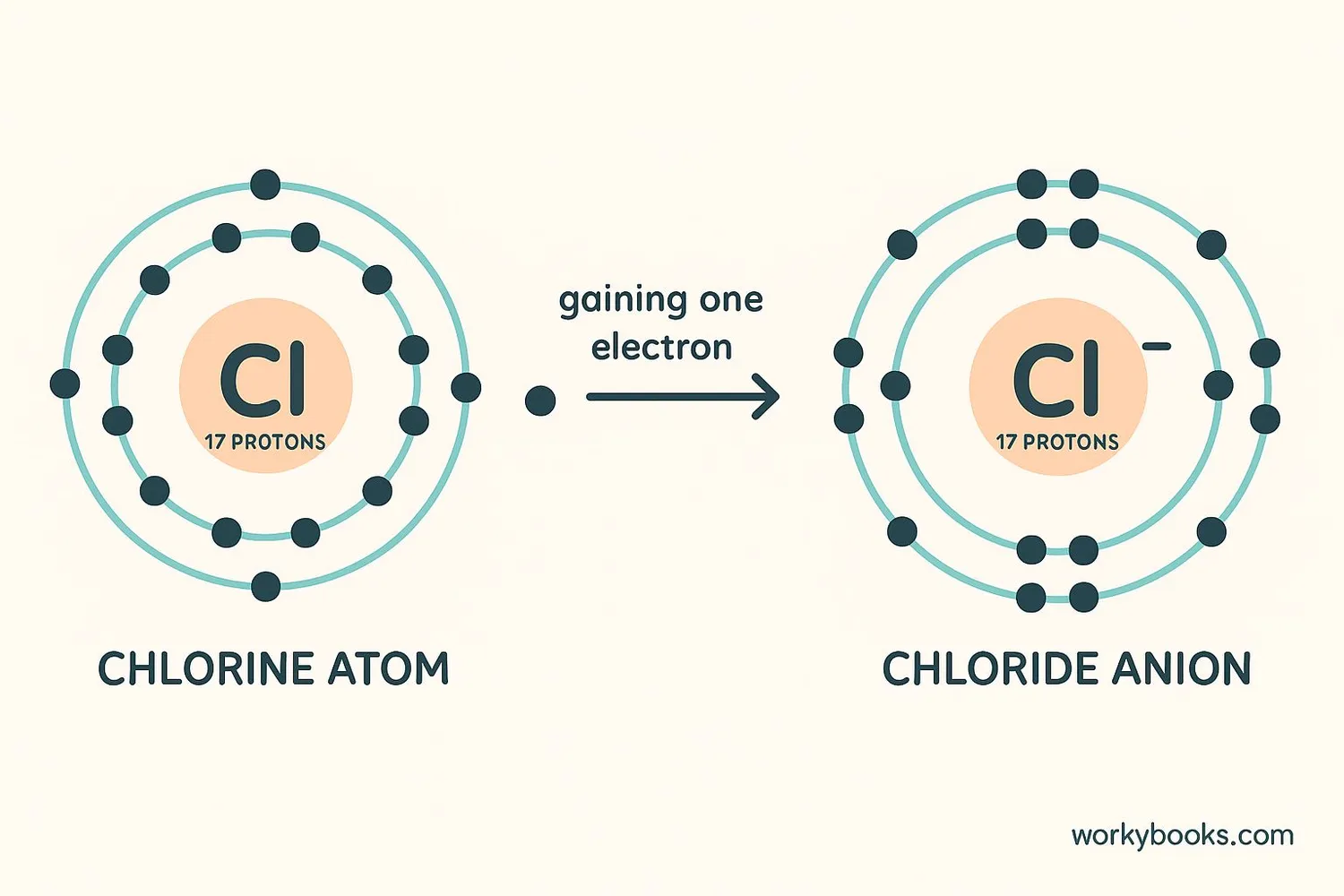
Anions form when atoms gain electrons. This process usually happens during chemical reactions. Let's see how it works:
Electron Gain
An atom gains one or more electrons from another atom
Charge Imbalance
Extra electrons create more negative charges than positive protons
Anion Formation
The atom becomes a negatively charged ion (anion)
Example: When a chlorine atom (Cl) gains an electron, it becomes a chloride anion (Cl⁻). This is why table salt is sodium chloride (NaCl) - it's made of sodium cations (Na⁺) and chloride anions (Cl⁻).
Non-metal atoms like oxygen, chlorine, and sulfur tend to form anions because they "want" to gain electrons to complete their outer electron shells.
Electron Affinity
The tendency of an atom to gain electrons is called electron affinity. Elements with high electron affinity form anions more easily.
Anions in Ionic Compounds
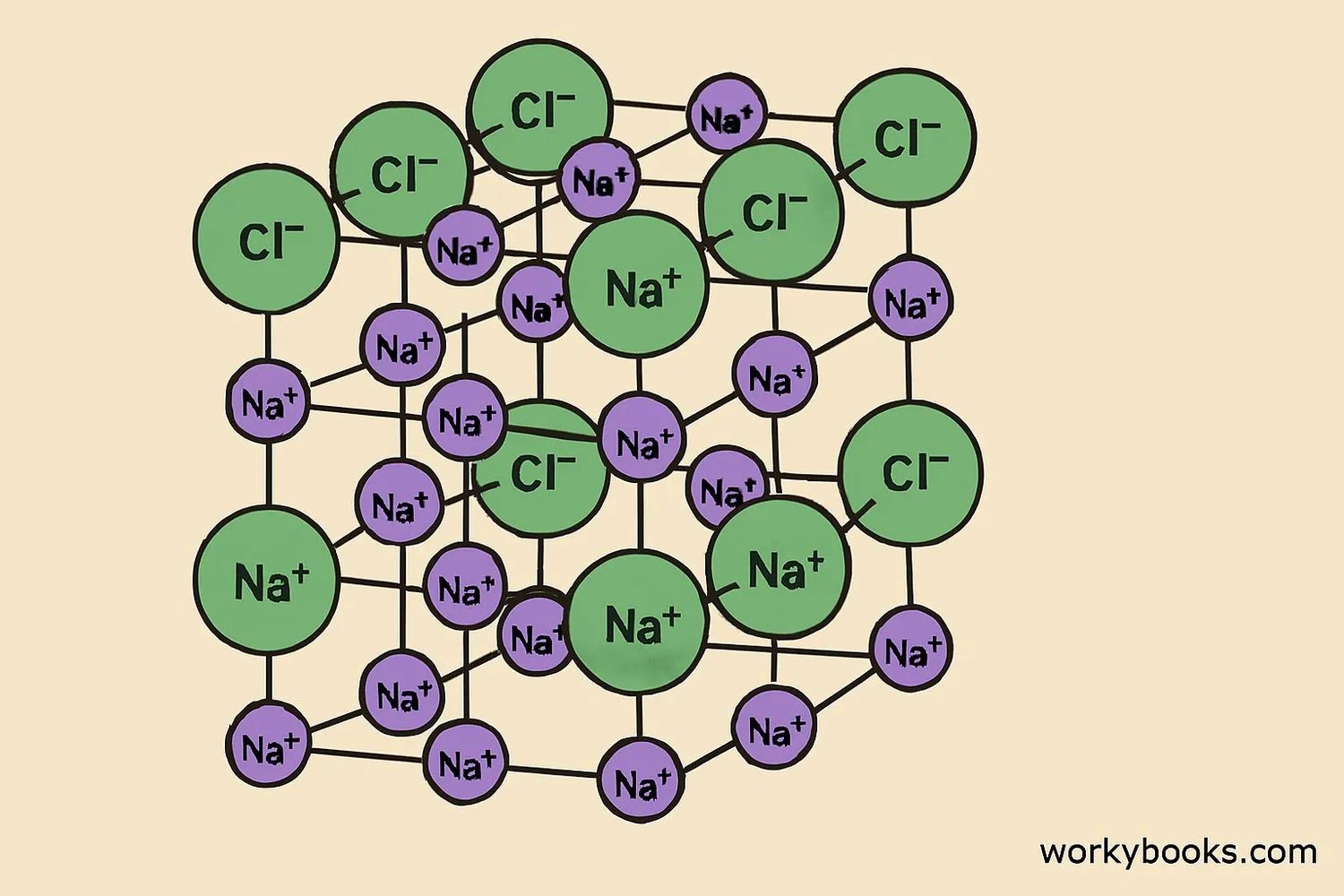
Anions play a crucial role in forming ionic compounds. These are substances made of positively charged cations and negatively charged anions held together by strong electrostatic forces called ionic bonds.
Here's how ionic compounds form:
Cation Formation
Metal atoms lose electrons to become positive cations
Anion Formation
Non-metal atoms gain electrons to become negative anions
Ionic Bonding
Opposite charges attract, forming ionic compounds
Properties of Ionic Compounds:
• Form crystal structures
• High melting and boiling points
• Conduct electricity when dissolved or molten
• Many are soluble in water
Common ionic compounds include table salt (NaCl), baking soda (NaHCO₃), and calcium carbonate (CaCO₃) found in chalk and limestone.
Anions in Daily Life
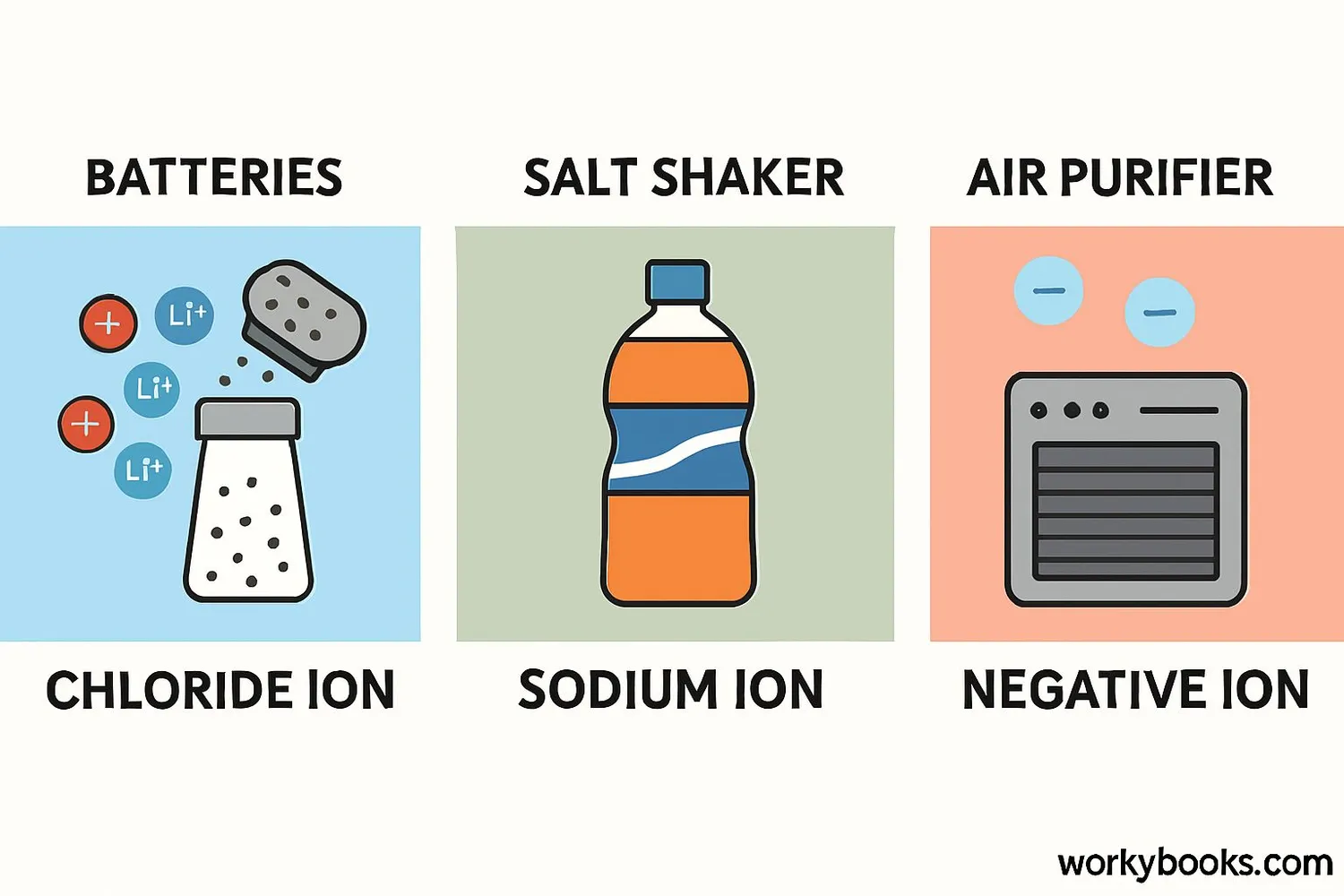
Anions are everywhere in our daily lives! They play important roles in many common substances and processes:
Batteries
Movement of ions between electrodes powers devices
Electrolytes
Anions in sports drinks help conduct electricity in our bodies
Air Purifiers
Negative ions attach to pollutants to clean the air
Electrolytes are substances that produce ions when dissolved in water. In our bodies, important electrolyte anions include:
• Chloride (Cl⁻) - helps maintain fluid balance
• Bicarbonate (HCO₃⁻) - regulates blood pH
• Phosphate (PO₄³⁻) - important for energy storage
These anions help conduct electrical signals in our nerves and muscles, making them essential for life!
Anion Quiz
Test your knowledge about anions with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about anions:
Anion Trivia
Discover some fascinating facts about anions:
Nature's Anions
The fresh smell after a thunderstorm comes from ozone (O₃) and negative ions created by lightning. These anions help clean the air by attaching to pollutants!
Ocean Air Benefits
Ocean air contains up to 2000 negative ions per cubic centimeter (compared to just 100-200 in city air). This is why ocean air feels so refreshing!
Salt Formation
When sodium (a metal) and chlorine (a gas) combine to form table salt, sodium loses an electron to become Na⁺ and chlorine gains that electron to become Cl⁻.
Body Electricity
Your nervous system uses ions (including anions) to transmit electrical signals at speeds up to 120 meters per second! This is how you feel touch and move muscles.





