Calorimetry - Definition, Examples, Quiz, FAQ, Trivia
Discover how scientists measure heat energy in chemical reactions and physical processes
What is Calorimetry?
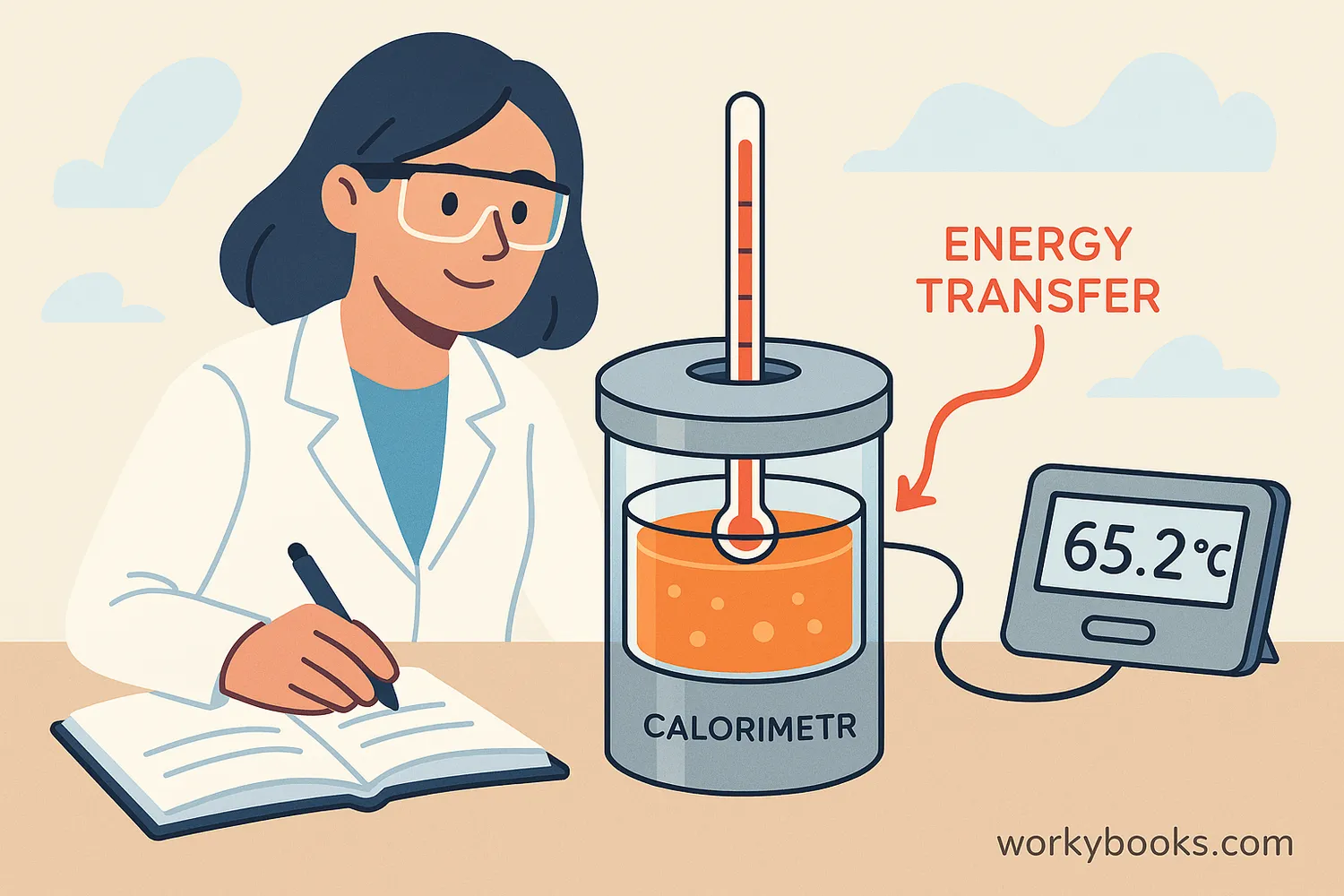
Calorimetry is the science of measuring heat transfer in chemical reactions or physical changes. It helps scientists understand how much energy is released or absorbed during these processes.
Think of calorimetry as a special way to "see" energy! When you burn a piece of wood or mix baking soda with vinegar, heat is either produced or absorbed. Calorimetry allows us to measure that heat energy precisely.
The word "calorimetry" comes from the Latin word "calor," which means heat, and the Greek word "metron," which means measure. So calorimetry literally means "heat measurement"!
Science Fact!
The first ice calorimeters were used in the 18th century by scientists like Joseph Black and Antoine Lavoisier to study heat transfer!
How Calorimetry Works
Calorimeters work by measuring temperature changes in a known amount of water or another substance. When a chemical reaction happens inside the calorimeter, it either releases heat (making things warmer) or absorbs heat (making things cooler).
Here's the basic process scientists use:
Contain
The reaction happens in an insulated container
Measure
Temperature changes are carefully measured
Calculate
Scientists calculate energy using the temperature change
The key formula used in calorimetry is:
Where:
• Q = heat energy (in joules)
• m = mass of the substance (in grams)
• c = specific heat capacity (how much energy it takes to heat 1 gram by 1°C)
• ΔT = change in temperature (in °C)
Real World Example
Nutrition labels on food packages use calorimetry! Food is burned in a calorimeter to measure how much energy it contains, which is reported as Calories.
Types of Calorimeters
Scientists use different types of calorimeters depending on what they need to measure:
Simple Calorimeter
Uses insulated cups to measure heat changes at constant pressure. Often used in school labs.
Bomb Calorimeter
Measures heat from combustion reactions at constant volume. Very precise for food energy measurements.
Differential Scanning Calorimeter
Measures how materials absorb heat as temperature changes. Used to study plastics, metals, and chemicals.
Each type of calorimeter has special features:
Simple calorimeters are like super-insulated coffee cups that prevent heat from escaping.
Bomb calorimeters have strong sealed containers that can handle high-pressure reactions.
Differential calorimeters can measure tiny differences in heat flow as temperature changes.
Why Calorimetry is Important
Calorimetry is used in many important scientific and industrial applications:
Food Science
Measuring Calorie content in foods and beverages for nutrition labels
Pharmaceuticals
Studying how drugs interact with the body and their stability
Material Science
Testing materials for safety, stability, and performance
Other important uses of calorimetry include:
• Environmental science: Studying heat effects in ecosystems
• Engineering: Testing fuel efficiency and energy production
• Chemistry: Understanding reaction energies and bonding
• Biology: Measuring metabolic rates in living organisms
Without calorimetry, we wouldn't have accurate nutrition information, safe medications, or many of the advanced materials we use every day!
Calorimetry Knowledge Check
Test your calorimetry knowledge with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about calorimetry:
Science Facts About Calorimetry
Discover some amazing facts about calorimetry and heat measurement!
Historical Origins
The first ice calorimeters were used in the 1780s by Antoine Lavoisier and Pierre-Simon Laplace. They measured the heat from guinea pig respiration by melting ice!
Precision Measurement
Modern calorimeters can measure temperature changes as small as 0.0001°C! This incredible sensitivity allows scientists to study very subtle energy changes.
Food Energy
The calorie values on nutrition labels are determined using bomb calorimeters. Food samples are burned completely, and the heat released is measured to calculate Calories.
Biological Calorimetry
Scientists use microcalorimeters to study tiny living things like bacteria and cells. They can measure the heat produced by metabolic processes in living organisms.





