Ethanol - Definition, Examples, Quiz, FAQ, Trivia
Discover how this renewable fuel is made from plants and used in our world
What is Ethanol?
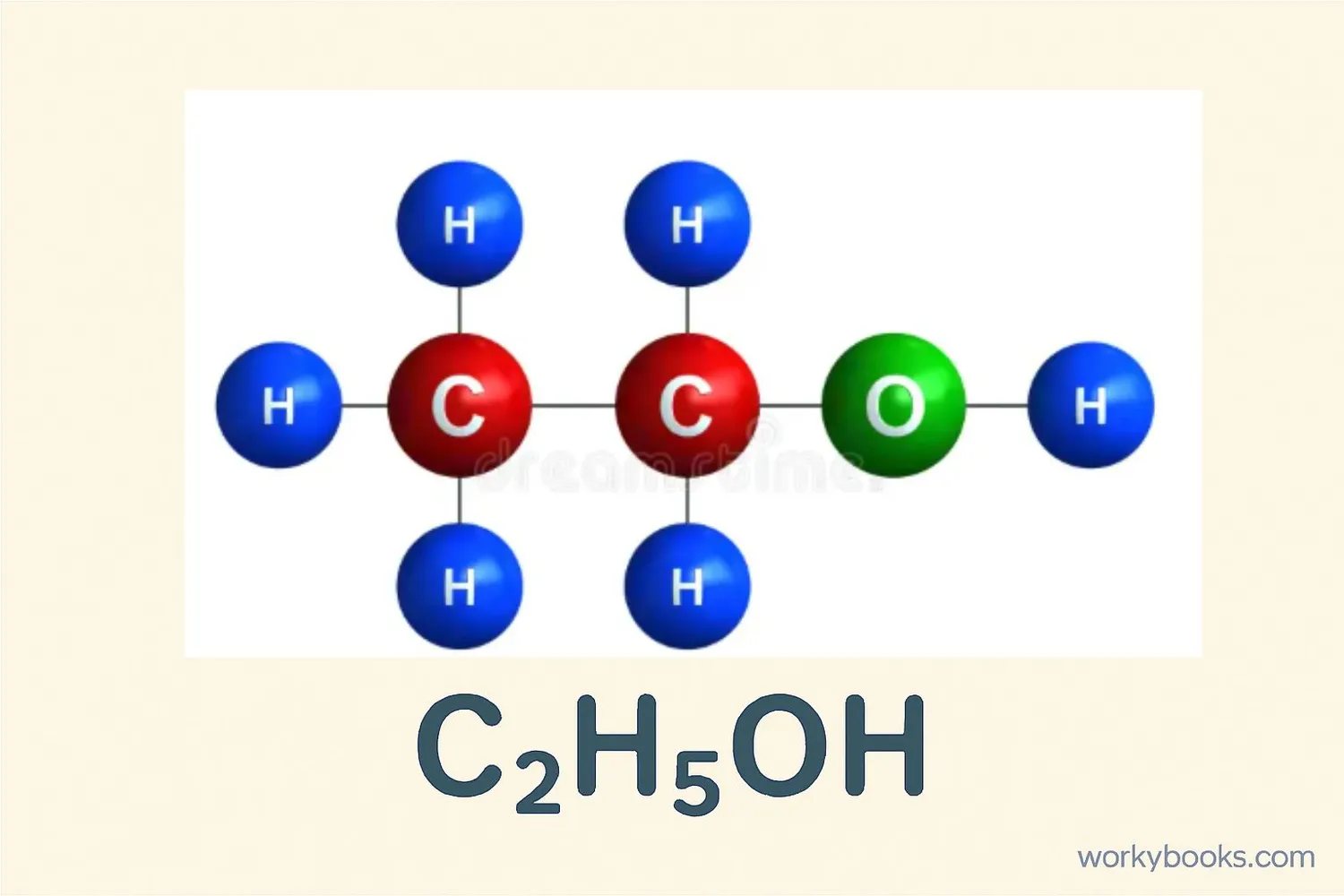
Ethanol is a clear, colorless liquid that is also known as ethyl alcohol. It's the same type of alcohol found in alcoholic beverages, but it has many other important uses too! Ethanol is a chemical compound made of carbon, hydrogen, and oxygen atoms.
The chemical formula for ethanol is:
Ethanol is a type of biofuel, which means it's made from plants rather than from fossil fuels like oil. This makes it a renewable fuel source that can help reduce pollution. Ethanol is also used in medicines, cleaning products, and as a solvent in many industries.
Did You Know?
Ethanol occurs naturally when yeast ferments sugars. This process has been used for thousands of years to make alcoholic beverages!
How Ethanol is Made
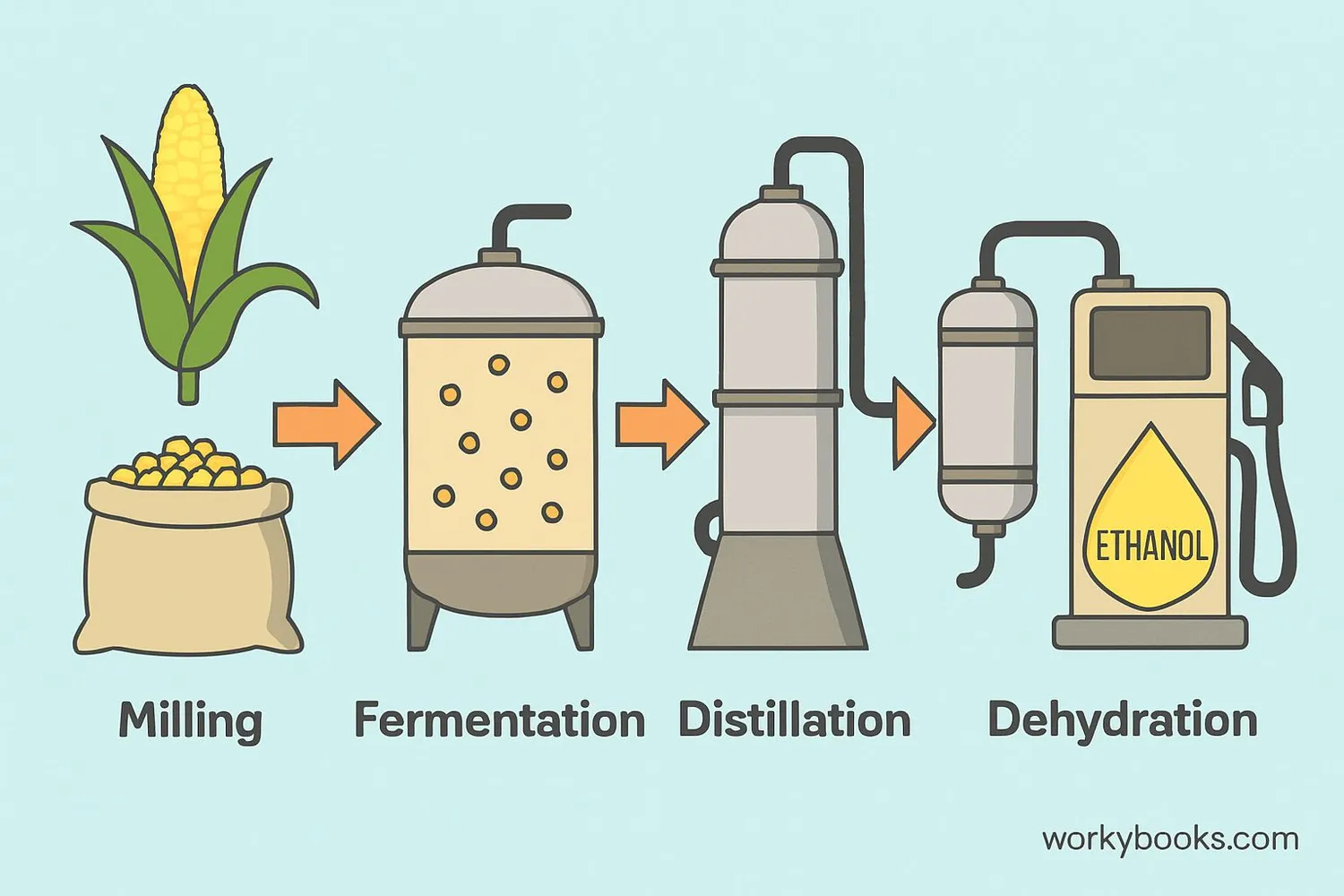
Ethanol is primarily made through a process called fermentation. This is how it works:
Raw Materials
Plants like corn, sugarcane, or wheat are harvested
Milling
The plants are ground up to release their sugars
Fermentation
Yeast is added to convert sugars into ethanol and CO₂
Distillation
The ethanol is separated from the mixture by heating
Dehydration
Water is removed to create fuel-grade ethanol
The fermentation process uses yeast to break down sugars. The chemical reaction looks like this:
C₆H₁₂O₆ → 2C₂H₅OH + 2CO₂
Which means: Sugar → Ethanol + Carbon Dioxide
Distillation Process
Distillation works because ethanol boils at a lower temperature (78°C) than water (100°C), allowing them to be separated.
Uses of Ethanol
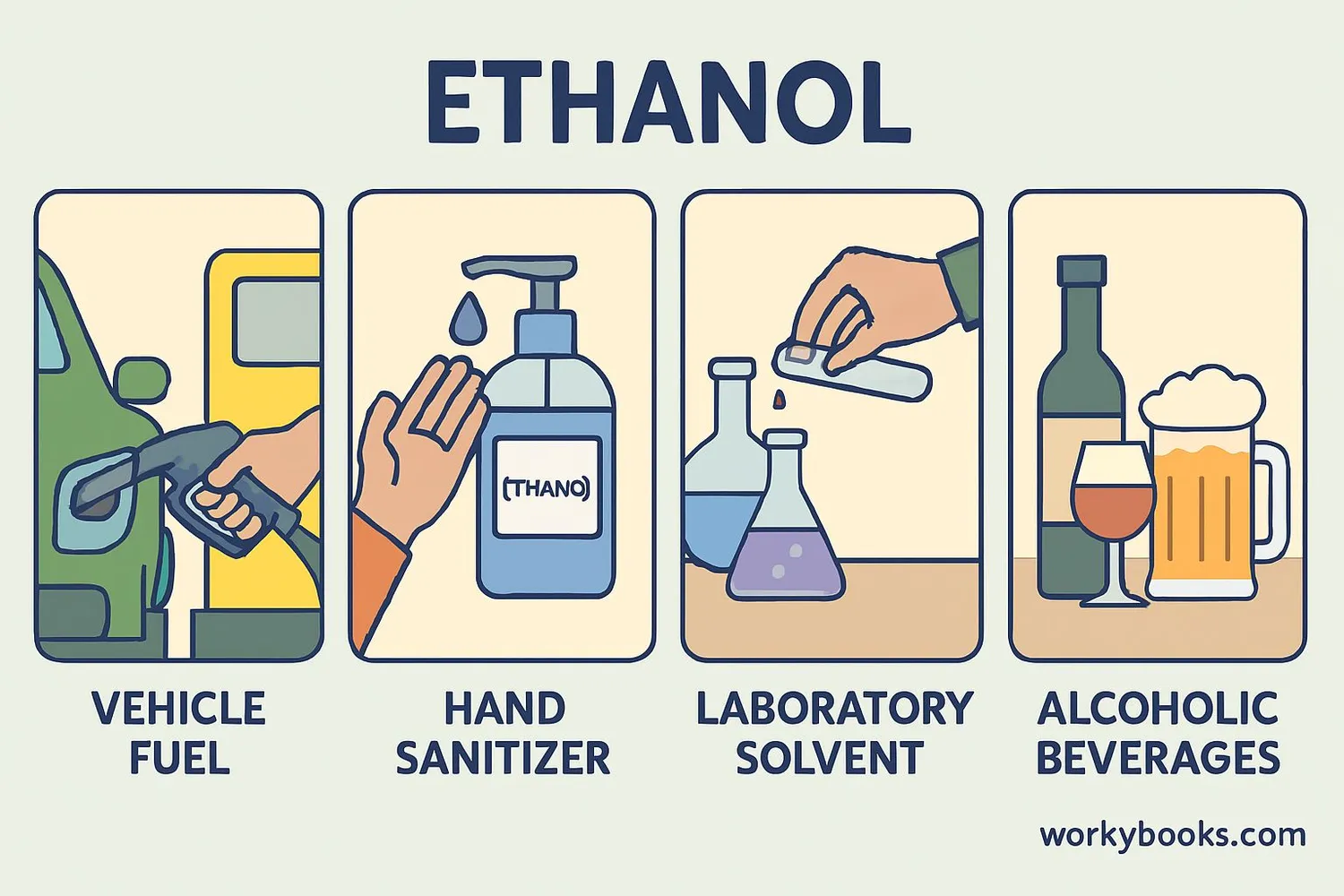
Ethanol has many important uses in our world:
Fuel
As a biofuel mixed with gasoline to power vehicles
Hand Sanitizer
Kills germs and bacteria in healthcare settings
Alcoholic Beverages
The intoxicating ingredient in beer, wine, and spirits
Solvent
Used in laboratories and industries to dissolve substances
Medicines
Used in medications like cough syrup and tinctures
As a fuel additive, ethanol helps:
• Reduce greenhouse gas emissions
• Increase octane rating for better engine performance
• Decrease dependence on fossil fuels
• Support agricultural economies
Most gasoline in the United States contains about 10% ethanol (E10), and some vehicles can run on up to 85% ethanol (E85).
Ethanol Knowledge Quiz
Test what you've learned about ethanol with this quiz! Answer all 5 questions to check your understanding.
Frequently Asked Questions
Here are answers to some common questions about ethanol:
Interesting Ethanol Facts
Discover some amazing facts about ethanol!
Ancient Origins
The production of ethanol through fermentation is one of the oldest organic reactions known to humanity. Evidence of alcoholic beverages dates back to 7000-6600 BCE in ancient China!
Energy Content
Ethanol contains about 34% less energy per gallon than gasoline. This is why vehicles running on high ethanol blends may get fewer miles per gallon than those running on pure gasoline.
Production Scale
The United States is the world's largest producer of ethanol, creating over 15 billion gallons annually. Brazil is second, producing about 8 billion gallons per year.
Freezing Point
Ethanol has a much lower freezing point (-173°F or -114°C) than water. This property makes it useful as an antifreeze in automotive applications and in laboratories.





