The pH Scale - Definition, Examples, Quiz, FAQ, Trivia
Discover how we measure acids and bases in our world
What is pH?
pH is a measure of how acidic or basic (alkaline) a solution is. The term "pH" stands for "potential of hydrogen" and measures the concentration of hydrogen ions (H⁺) in a solution.
Think of pH as a way to describe how much acid is in something. When hydrogen ions are high, the solution is acidic. When hydrogen ions are low, the solution is basic or alkaline.
Water is the perfect balance point! Pure water has a pH of 7, which we call neutral - not acidic or basic.
Chemistry Fact!
pH is measured on a scale from 0 to 14, with lower numbers being more acidic and higher numbers more basic.
Measuring pH
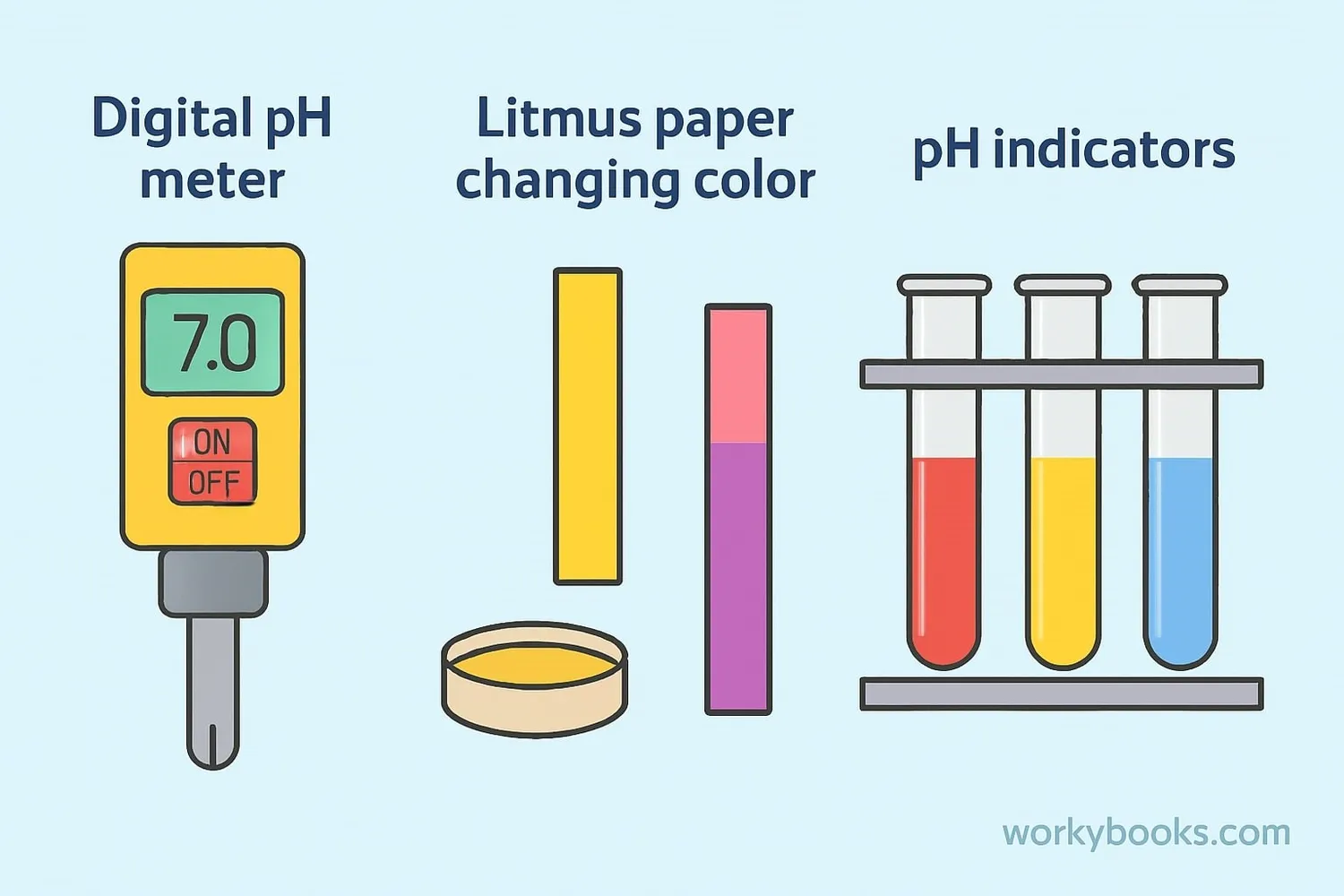
Scientists have developed several ways to measure pH. Here are the most common methods:
Litmus Paper
Special paper that changes color when dipped in acidic or basic solutions
pH Meters
Electronic devices that give a precise pH reading when placed in a solution
pH Indicators
Liquid chemicals that change color based on the pH of a solution
Litmus paper is the simplest method - it turns red in acids and blue in bases. pH meters are more accurate and used in laboratories. pH indicators like phenolphthalein or bromothymol blue can show a range of pH values through different color changes.
Did You Know?
Some natural substances like red cabbage juice can be used as pH indicators! It turns pink in acids and green in bases.
The pH Scale
The pH scale ranges from 0 to 14. Here's what each part of the scale means:
0-3: Strong Acid
Examples: Battery acid, stomach acid
4-6: Weak Acid
Examples: Coffee, milk, rainwater
7: Neutral
Examples: Pure water
8-10: Weak Base
Examples: Sea water, baking soda
11-14: Strong Base
Examples: Soapy water, drain cleaner
Each whole number on the pH scale represents a 10-fold difference in acidity or basicity. For example, a pH of 3 is 10 times more acidic than a pH of 4, and 100 times more acidic than a pH of 5!
Why pH Matters
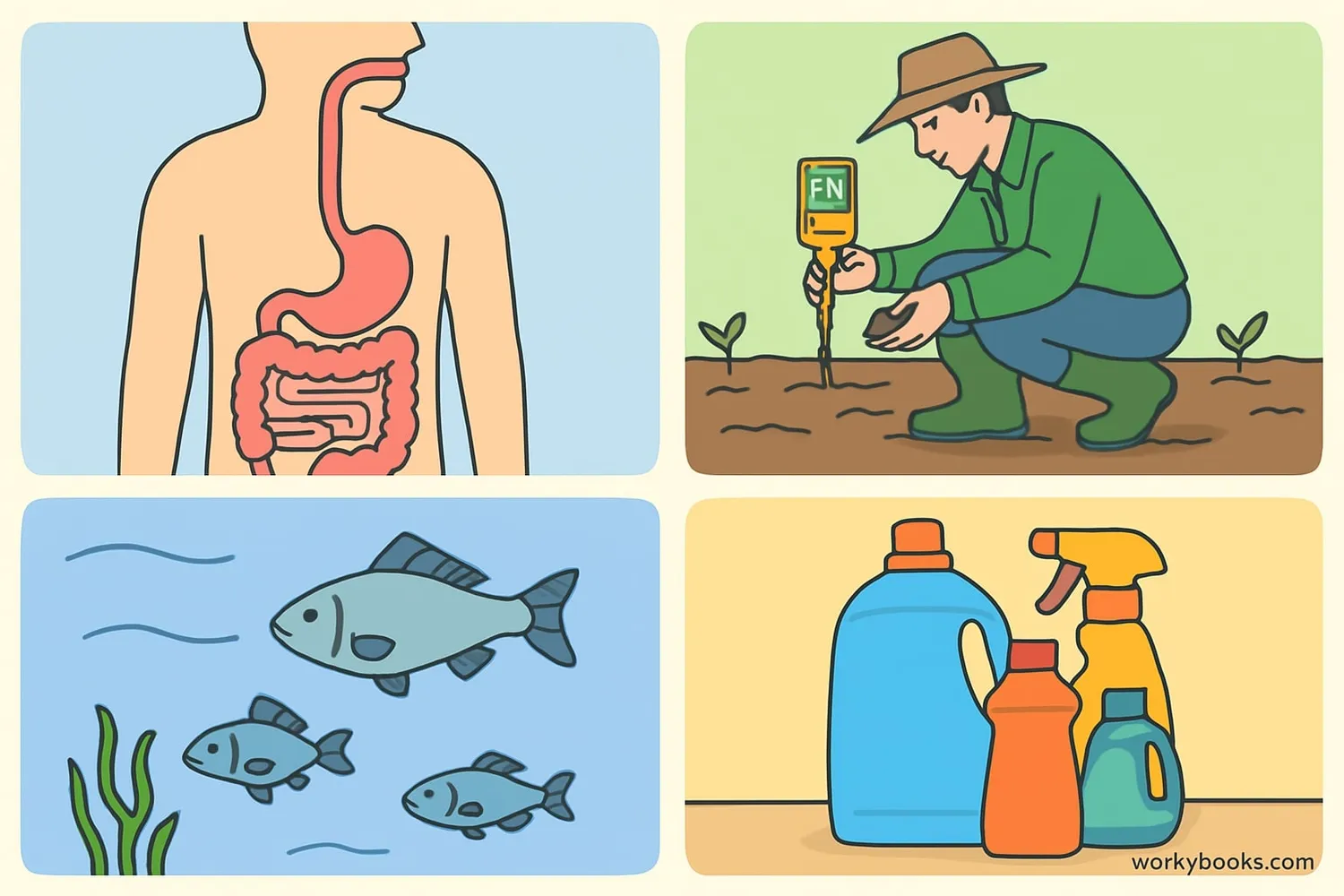
pH is important in many areas of our lives and the natural world:
Human Health
Our blood has a pH around 7.4 - even small changes can make us sick!
Agriculture
Plants need specific pH levels to absorb nutrients from soil
Environment
Aquatic life requires specific pH levels to survive in lakes and rivers
Household Products
Cleaning products use pH properties to be effective
Maintaining proper pH balance is essential for:
• Our body's chemical reactions
• Healthy plant growth in gardens and farms
• Survival of fish and other aquatic organisms
• Effectiveness of medicines and cleaning products
• Preventing corrosion in pipes and machinery
pH Scale Quiz
Test your pH knowledge with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about pH:
Fun pH Trivia
Discover some amazing facts about pH!
Extreme pH Levels
The strongest acid ever created is fluoroantimonic acid, which is 10 quadrillion times stronger than sulfuric acid! The strongest base is ortho-diethynylbenzene dianion.
Body pH Variations
Different parts of your body have different pH levels! Stomach acid is pH 1.5-3.5, skin is pH 4-7, and pancreatic fluid is pH 8-8.3.
Ocean Acidification
Oceans have absorbed about 30% of human-produced CO₂, causing ocean pH to decrease by 0.1 units since the Industrial Revolution. This affects marine life with calcium carbonate shells.
pH History
The pH scale was invented in 1909 by Danish chemist Søren Sørensen while working at the Carlsberg Laboratory in Copenhagen. He needed to measure acidity during beer brewing!





