Sublimation - Definition, Examples, Quiz, FAQ, Trivia
Discover how solids can transform directly into gases!
What is Sublimation?
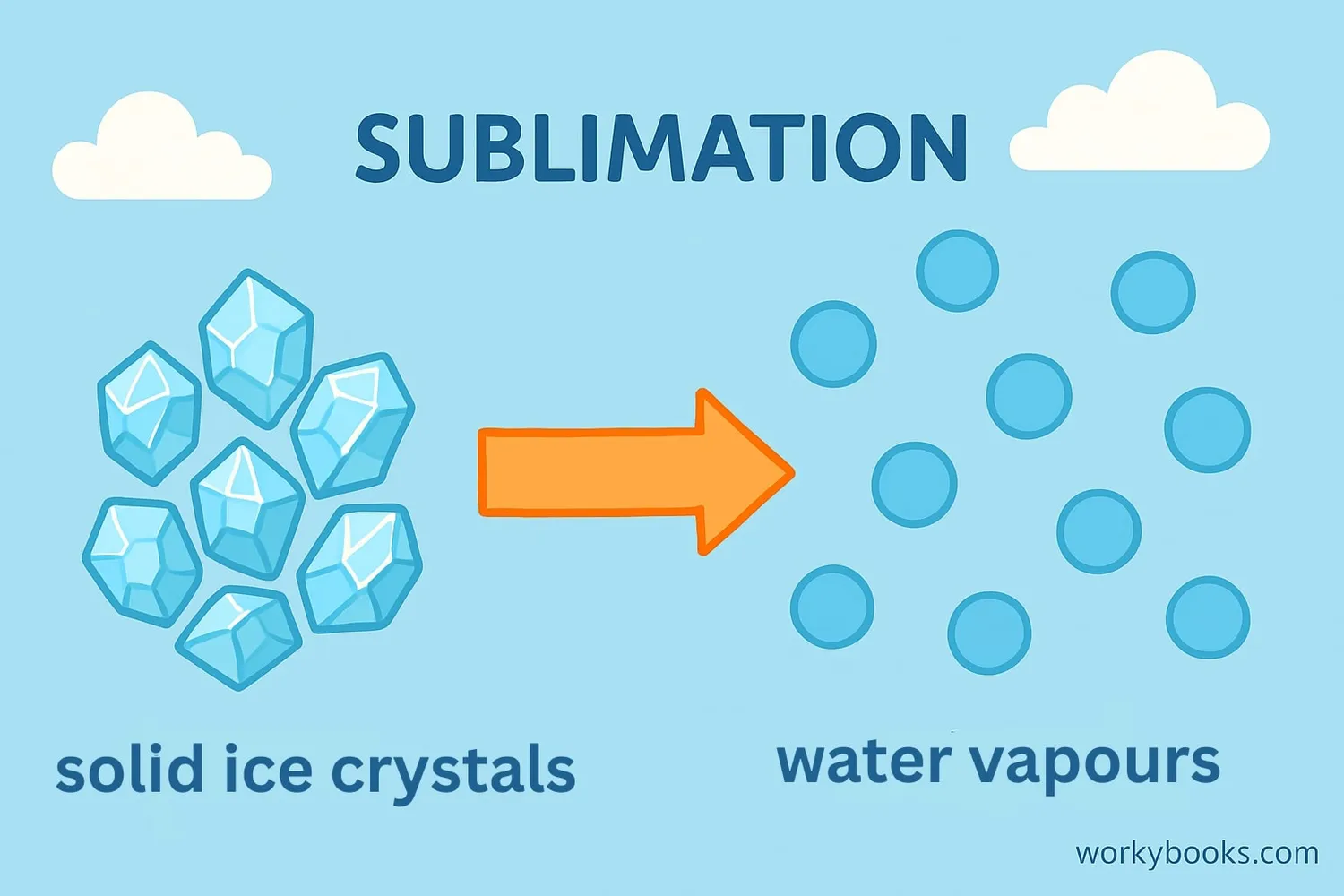
Sublimation is a fascinating process where a solid turns directly into a gas without ever becoming a liquid. It's a special kind of phase transition that happens when certain solids get warm enough to skip the liquid state entirely.
Think of an ice cube. Normally, when it warms up, it melts into water (liquid) and then evaporates into vapor (gas). But with sublimation, solids like dry ice or snow in very cold places transform directly from solid to gas! This happens because the molecules gain enough energy to break free from the solid structure without passing through the liquid phase.
Science Fact!
At normal atmospheric pressure, water ice can sublimate when the temperature is below freezing and the air is very dry!
How Sublimation Works
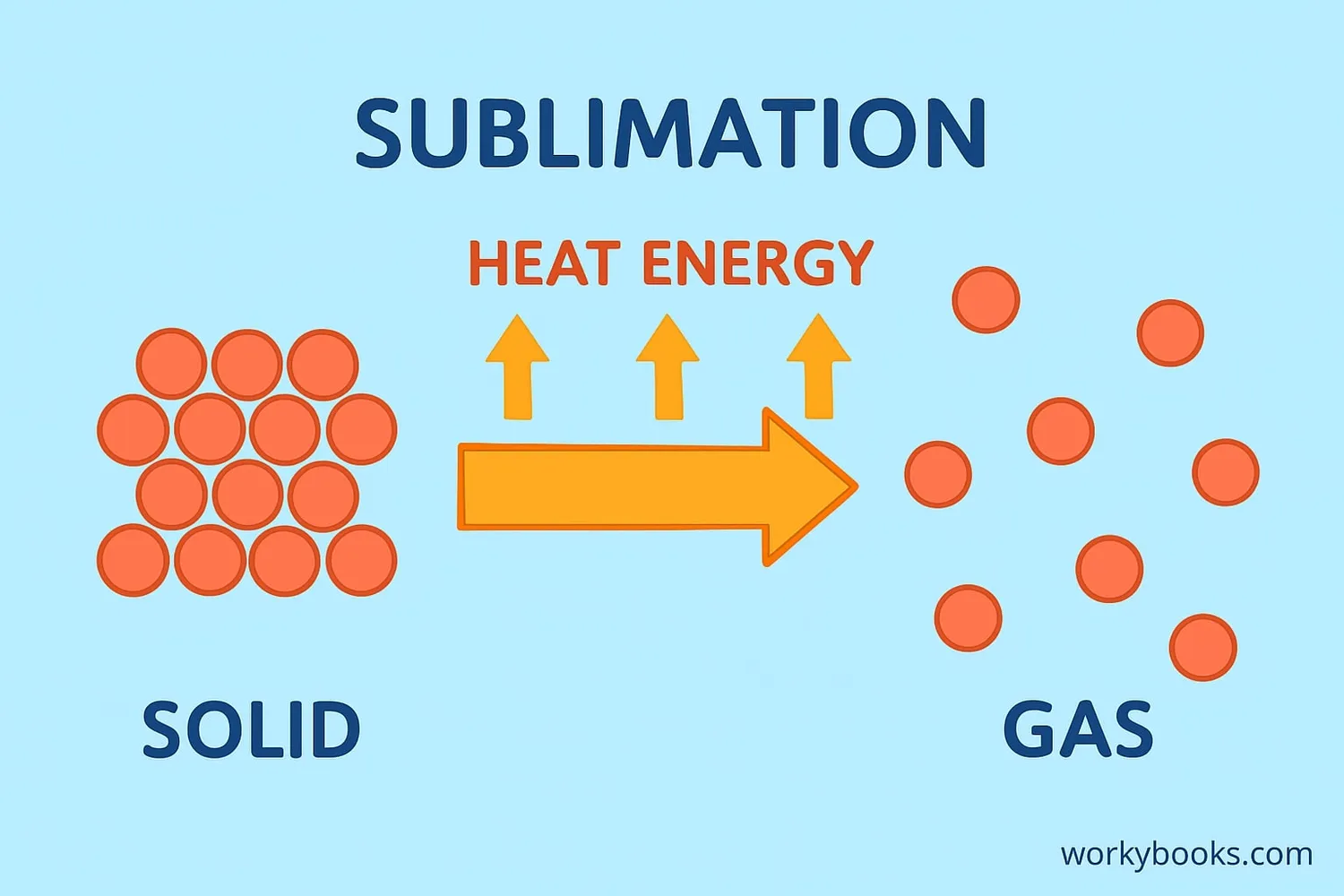
Sublimation happens because of the way molecules behave when they gain energy. Here's the science behind it:
Heat Energy
Heat is added to a solid substance
Molecular Motion
Molecules gain energy and vibrate faster
Breaking Bonds
Molecules break free from the solid structure
Gas Formation
Molecules form a gas without becoming liquid
Reverse Process
Deposition: Gas turns directly to solid
The heat of sublimation is the energy required for a substance to change directly from solid to gas. Different substances have different sublimation temperatures and pressures. The triple point is the specific temperature and pressure where all three phases (solid, liquid, gas) exist in balance.
Triple Point!
Water's triple point occurs at 0.01°C (32.02°F) and 611.657 pascals of pressure. At this point, ice, water, and vapor can all exist together!
Examples of Sublimation
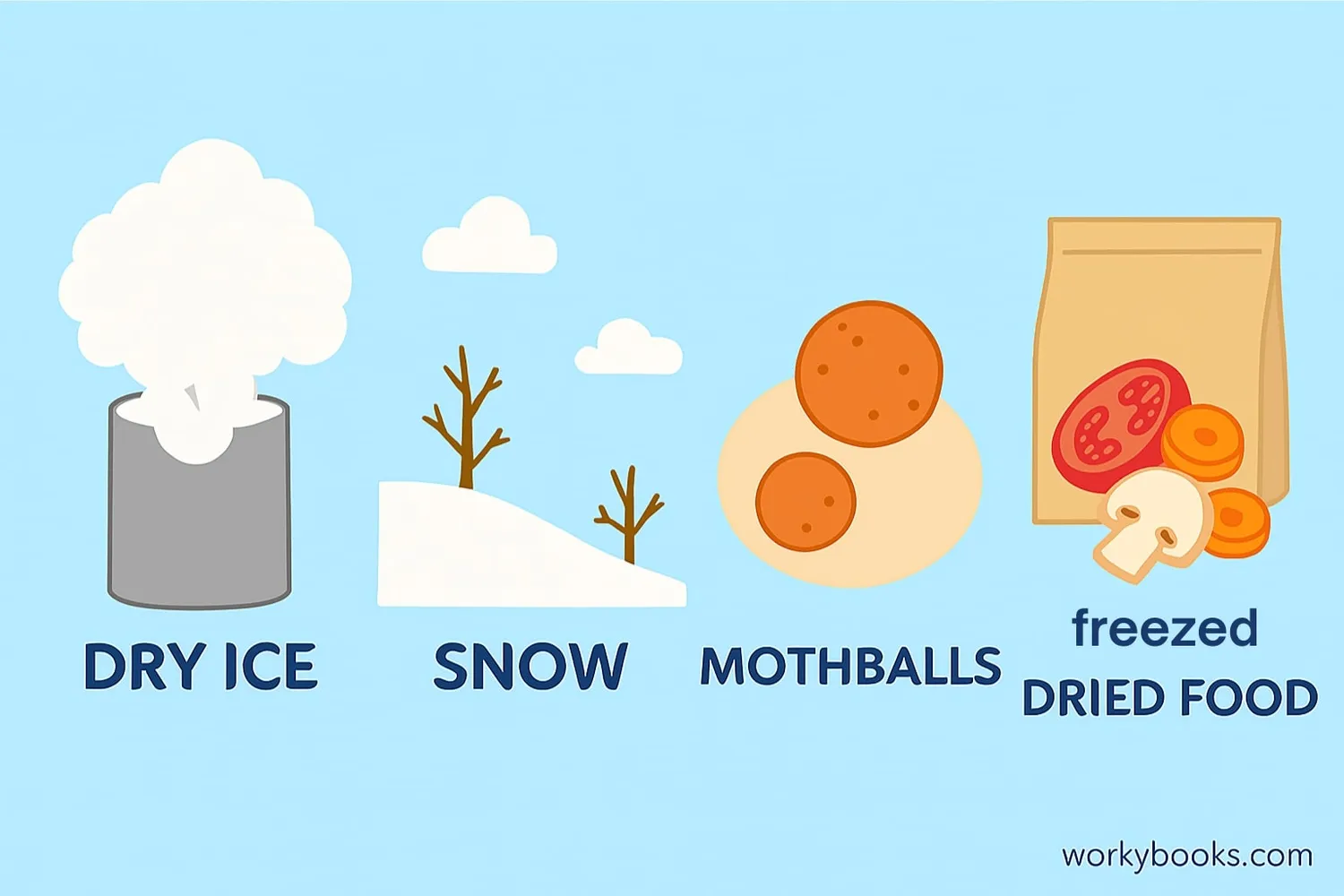
Sublimation isn't just a science concept - it happens all around us! Here are some common examples:
Dry Ice
Solid carbon dioxide sublimes at -78.5°C, creating fog effects
Snow & Ice
In cold, dry conditions, snow disappears without melting
Mothballs
Naphthalene balls sublime to release gas that repels insects
Freeze-Drying
Food preservation technique that uses sublimation
Dry ice sublimation is particularly fascinating because it creates dramatic fog effects. When dry ice (solid CO₂) is exposed to room temperature, it doesn't melt - it directly turns into carbon dioxide gas. This process absorbs heat from the surroundings, causing water vapor in the air to condense into fog.
Sublimation vs Evaporation
While both processes result in a gas, sublimation and evaporation are different:
| Feature | Sublimation | Evaporation |
|---|---|---|
| Starting Phase | Solid | Liquid |
| Ending Phase | Gas | Gas |
| Intermediate Phase | None (direct change) | Liquid phase exists |
| Common Examples | Dry ice, mothballs, snow in cold air | Water drying, sweat evaporating |
| Energy Required | Heat of sublimation | Heat of vaporization |
Energy Comparison
The heat of sublimation is equal to the sum of the heat of fusion (solid to liquid) and the heat of vaporization (liquid to gas). This is why sublimation requires more energy than evaporation alone!
Uses of Sublimation

Sublimation isn't just a natural phenomenon - humans have found clever ways to use it:
Sublimation Printing
Special inks turn to gas and bond with polyester fabrics
Pharmaceuticals
Purifying chemicals through sublimation
Freeze-Drying
Preserving food, medicines, and biological samples
Sublimation printing is especially popular for creating custom t-shirts, mugs, and other products. The process uses special dyes that turn into gas when heated, then bond with polymer materials. This creates vibrant, long-lasting prints that won't crack or peel like traditional prints.
Sublimation Quiz
Test your knowledge about sublimation with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about sublimation:
Fun Sublimation Trivia
Discover some amazing facts about sublimation:
Comet Tails
Comets develop their spectacular tails through sublimation! When comets approach the sun, their icy surfaces sublime, releasing gas and dust that form the characteristic tails we see from Earth.
Antarctica's Dry Valleys
In Antarctica's Dry Valleys, the air is so dry that ice sublimes rather than melts. These valleys are the closest Earth equivalent to the surface of Mars!
Art Conservation
Museums use sublimation to preserve valuable documents. Freeze-drying through sublimation removes moisture from water-damaged papers and artifacts without causing further damage.
Instant Coffee
Your instant coffee was likely made using sublimation! Freeze-drying preserves the coffee's flavor and aroma better than other drying methods, and it's done through sublimation.





