Ocean Acidification - Definition, Examples, Quiz, FAQ, Trivia
Learn how carbon dioxide changes ocean chemistry and affects marine life
What is Ocean Acidification?

Ocean acidification is the process where our oceans become more acidic because they absorb too much carbon dioxide (CO2) from the air. Just like how soda becomes fizzy when CO2 is added, our oceans are changing because of extra CO2.
The ocean is like Earth's giant sponge. It absorbs about 30% of the CO2 we release by burning fossil fuels (like coal, oil, and gas). This extra CO2 changes the chemistry of seawater, making it more acidic. Scientists measure acidity using the pH scale - lower numbers mean more acidic conditions.
Did You Know?
The ocean is now 30% more acidic than it was before the Industrial Revolution 200 years ago. That's the fastest change in ocean chemistry in 50 million years!
Human Activities
Burning fossil fuels releases CO2 into the atmosphere
CO2 Absorption
Oceans absorb about 30% of this excess CO2
Chemical Change
CO2 reacts with seawater to form carbonic acid
How Ocean Acidification Happens
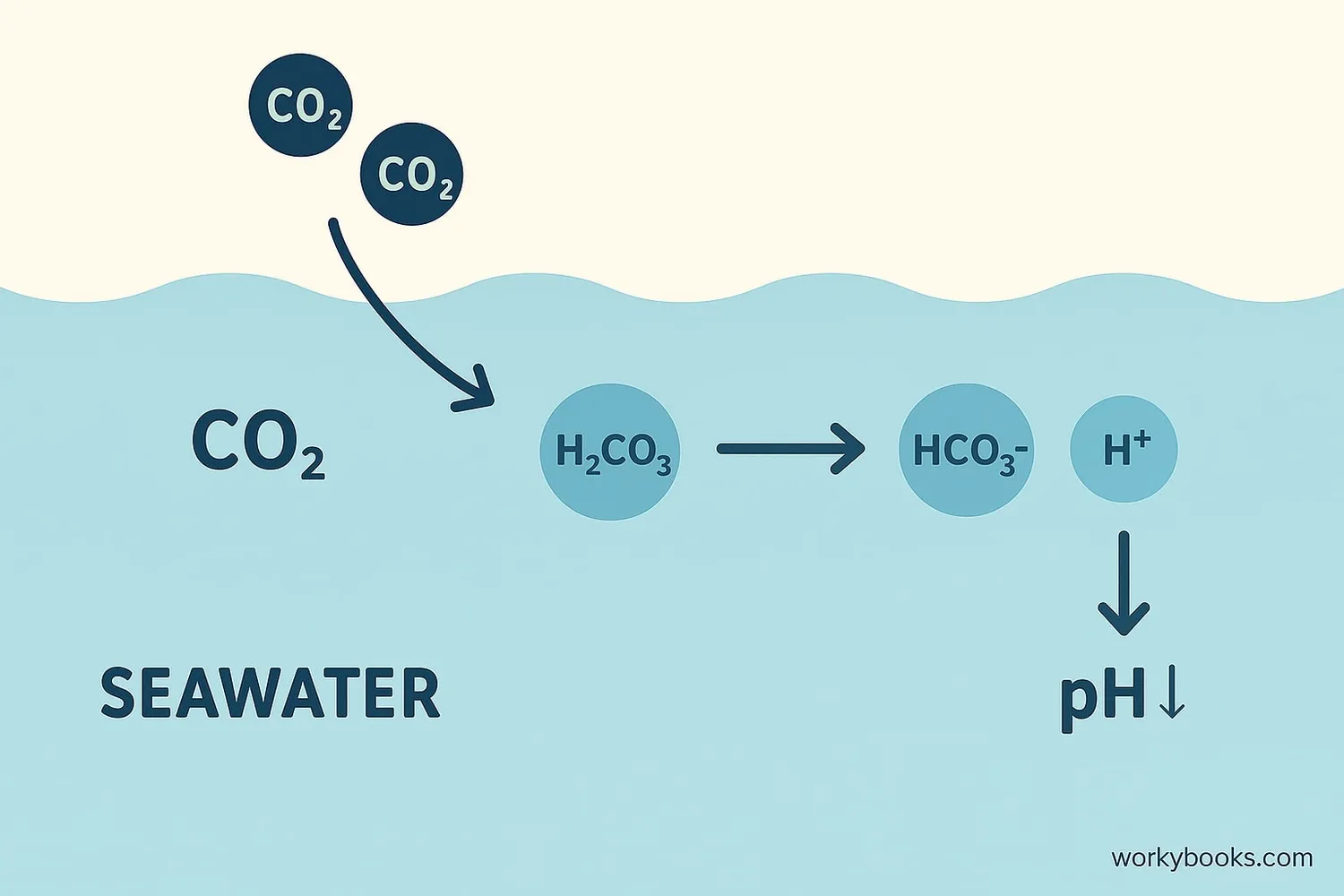
When carbon dioxide (CO2) dissolves in seawater, it sets off a chemical reaction:
CO2 Enters Water
CO2 from the air dissolves into ocean water
Forms Carbonic Acid
CO2 + H2O → H2CO3 (carbonic acid)
Breaks Down
Carbonic acid breaks into bicarbonate and hydrogen ions
Hydrogen Ions
More hydrogen ions (H+) make water more acidic
The increase in hydrogen ions is what makes the ocean more acidic. This process reduces the availability of carbonate ions that many marine organisms need to build their shells and skeletons.
pH Scale
Normal ocean pH is about 8.1 (slightly basic). Since the Industrial Revolution, it has dropped to 8.0. While this might seem small, the pH scale is logarithmic, meaning a 0.1 decrease represents a 30% increase in acidity!
Impacts on Marine Life
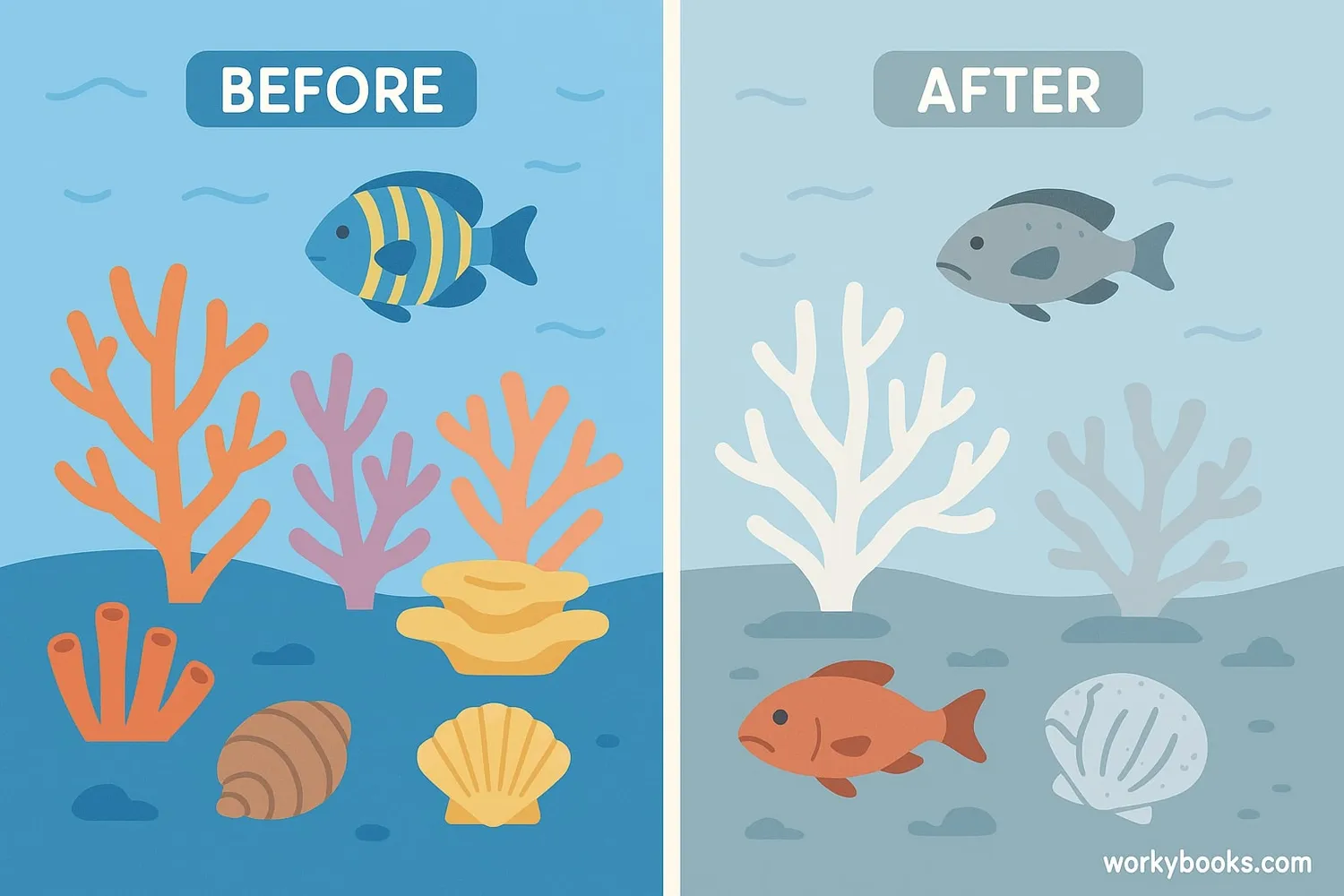
Ocean acidification affects marine life in different ways, especially organisms that build shells or skeletons from calcium carbonate:
Shellfish
Oysters, clams, and mussels struggle to build and maintain their shells
Corals
Reef-building corals grow more slowly and their skeletons weaken
Pteropods
Tiny "sea butterflies" have shells that dissolve in acidic waters
Fish Behavior
Some fish have trouble smelling predators or finding habitats
These changes ripple through the entire food chain. Small organisms like pteropods are food for fish, salmon, and whales. When they're affected, larger animals struggle to find enough food. Coral reefs provide habitat for 25% of marine species, so their decline affects countless ocean creatures.
Coral Reef Crisis
By 2050, 90% of coral reefs could experience severe bleaching events each year if CO2 emissions continue at current rates. This threatens the 500 million people who depend on reefs for food and coastal protection.
Ocean Acidification Quiz
Test your knowledge about ocean acidification with this 5-question quiz!
Frequently Asked Questions
Here are answers to common questions about ocean acidification:
Ocean Acidification Trivia
Discover fascinating facts about our changing oceans:
Historical Change
The ocean is currently acidifying 10 times faster than during any period in the last 55 million years. Marine life today has less time to adapt to these rapid changes.
The Pteropod Problem
Pteropods, tiny "sea butterflies," are dissolving in some Antarctic waters. These creatures are a vital food source for salmon, herring, and whales, making them a critical link in ocean food chains.
Coral Construction
Corals in acidic waters grow more slowly and their skeletons become weaker. By 2100, coral reefs could erode faster than they can rebuild if emissions continue at current rates.
Economic Impact
The U.S. shellfish industry could lose over $400 million annually by 2100 due to ocean acidification. Oyster hatcheries in the Pacific Northwest have already experienced significant losses.





