Beta Decay - Definition, Examples, Quiz, FAQ, Trivia
Discover how unstable atoms transform and release energy!
What is Beta Decay?
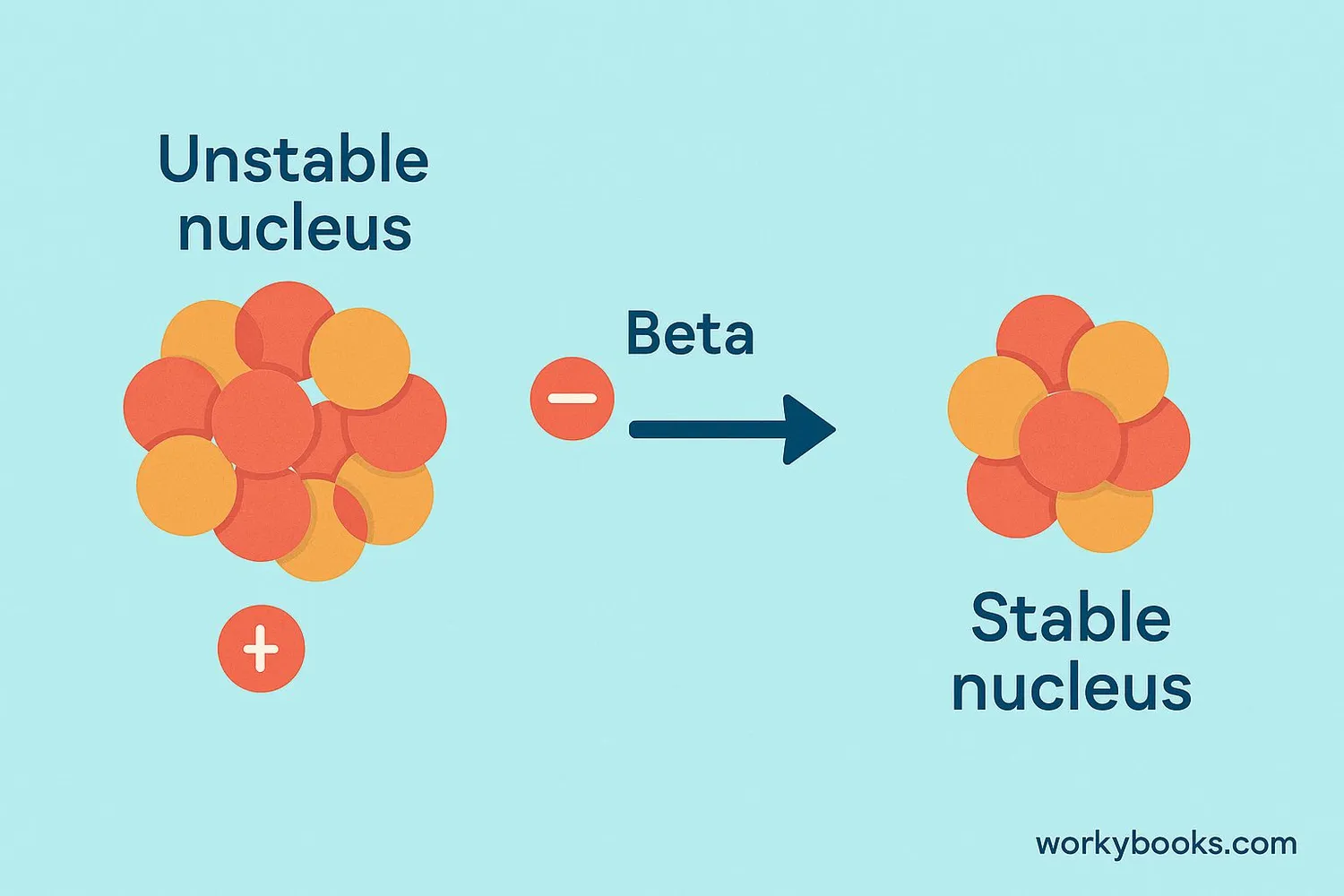
Beta decay is a type of radioactive decay where an unstable atomic nucleus transforms by emitting a beta particle. This happens when an atom has too many neutrons or protons to be stable. To become more stable, it releases a tiny particle called a beta particle and changes into a different element!
Think of it like this: Atoms want to be stable and balanced. When they have too many neutrons, they can "spit out" an electron to become more stable. This process transforms the atom into a different element!
Science Fact!
Beta decay occurs naturally in elements like carbon-14 and potassium-40, which are found in our environment and even in our bodies!
How Beta Decay Works
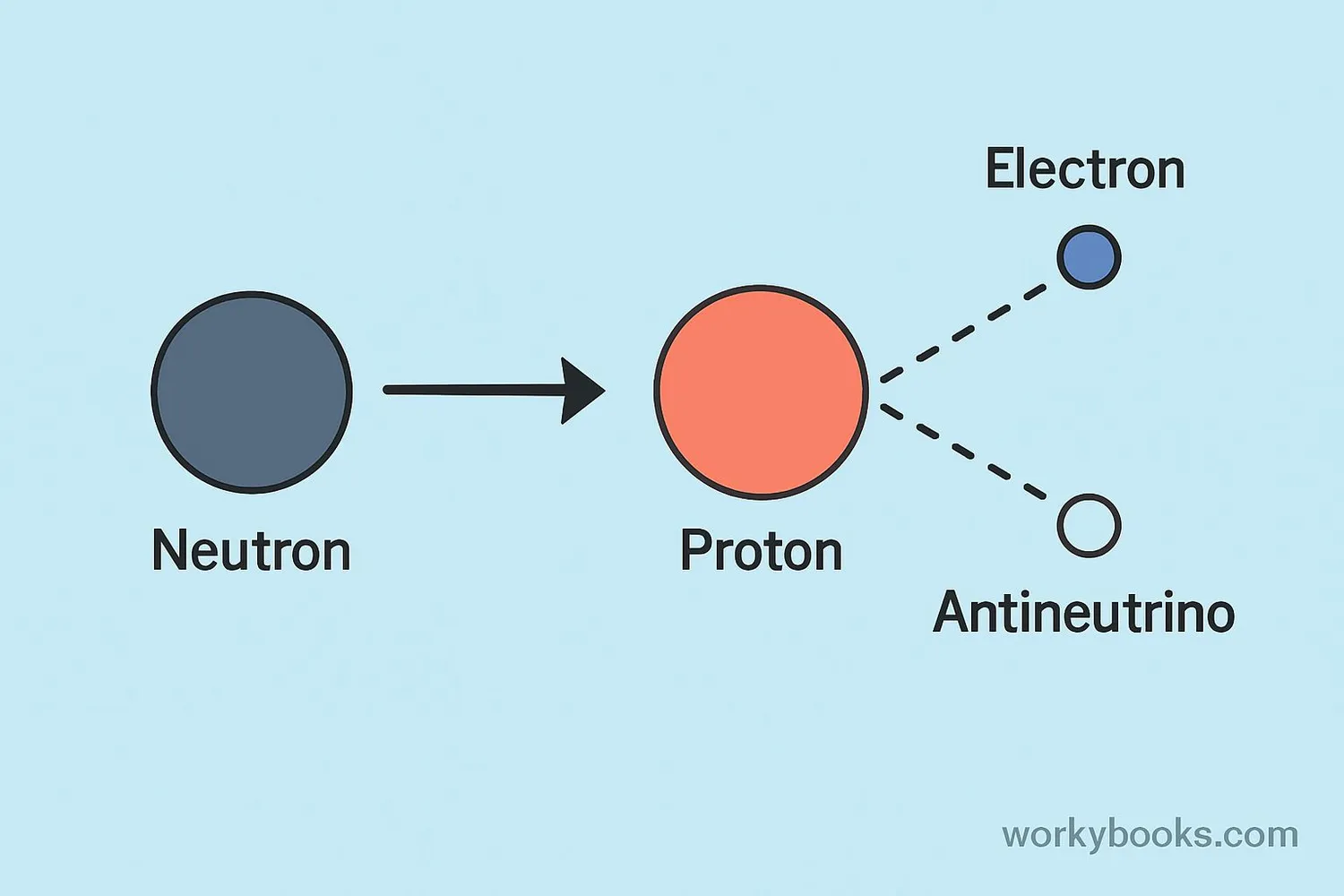
Beta decay happens when a neutron in an unstable nucleus transforms into a proton, or a proton transforms into a neutron. This transformation releases energy in the form of radiation. Here's what happens step by step:
Unstable Nucleus
The atom has too many neutrons to be stable
Neutron Transformation
A neutron changes into a proton
Beta Particle Emission
An electron (beta particle) is emitted
Antineutrino Release
A nearly massless antineutrino is released
New Element Formed
The atom becomes a different element
The special equation for beta-minus decay is:
Radiation Fact!
Beta particles can be stopped by a sheet of aluminum or even thick clothing, but they can be dangerous if radioactive materials are ingested.
Types of Beta Decay
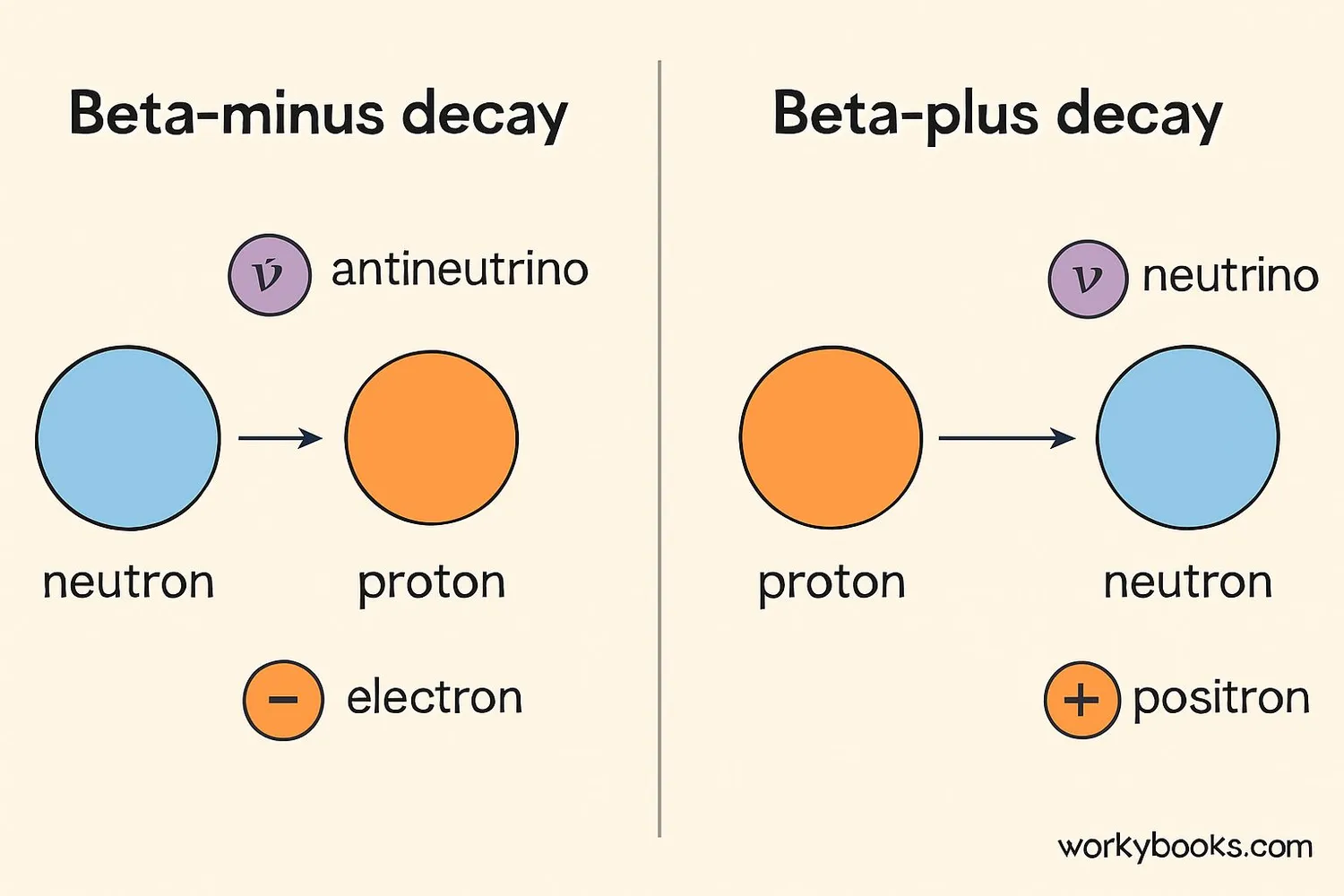
There are two main types of beta decay, each with different particle emissions:
Beta-Minus Decay (β⁻)
This happens when a nucleus has too many neutrons. A neutron transforms into a proton, emitting an electron and an antineutrino.
Example: Carbon-14 decaying to Nitrogen-14
Beta-Plus Decay (β⁺)
This happens when a nucleus has too many protons. A proton transforms into a neutron, emitting a positron and a neutrino.
Example: Fluorine-18 decaying to Oxygen-18
Medical Application!
Beta-plus decay is used in PET scans (medical imaging) where radioactive substances emit positrons that create gamma rays when they meet electrons.
Why Beta Decay is Important
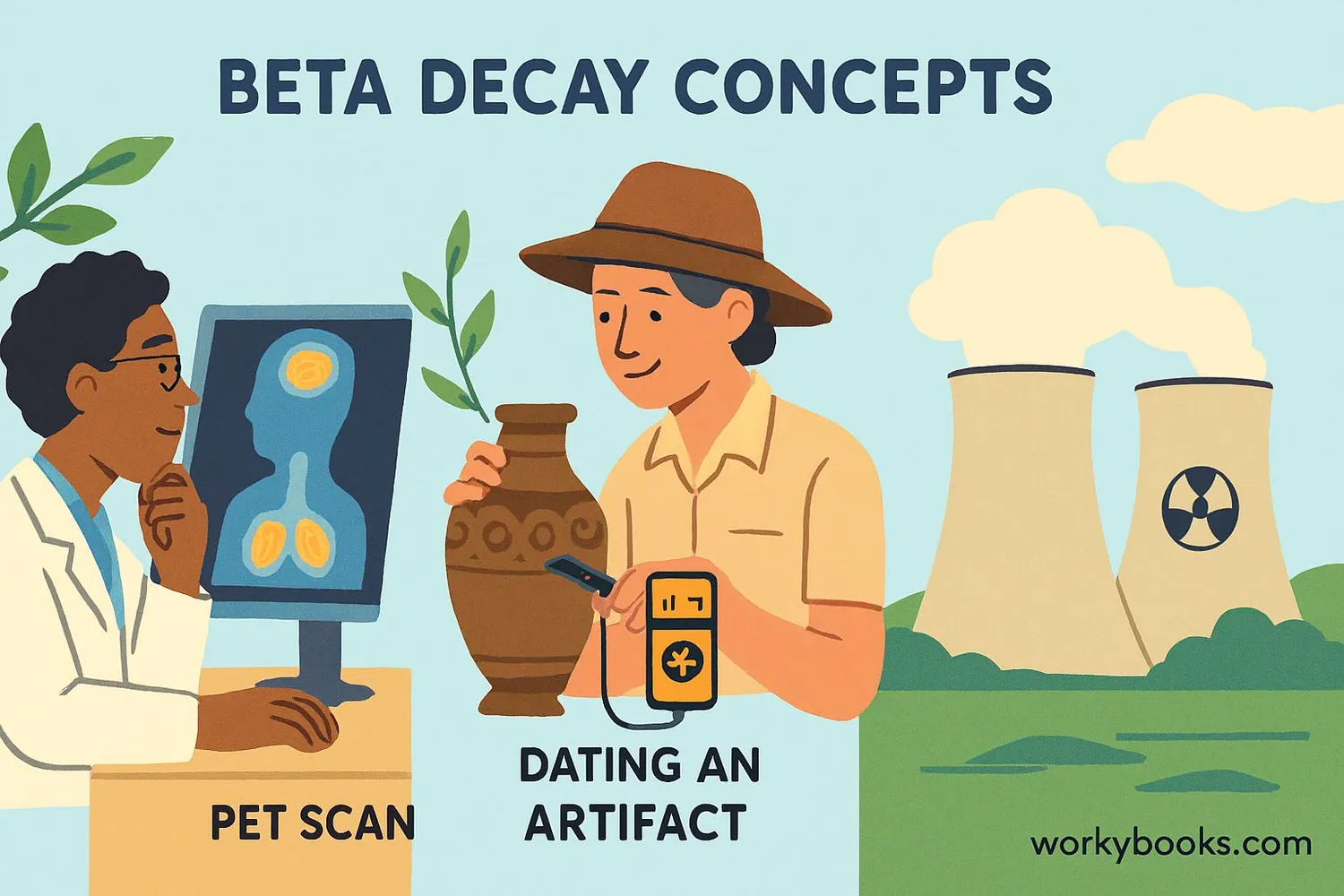
Beta decay plays a crucial role in science and our daily lives. Here's why it matters:
Medical Applications
Used in cancer treatments and medical imaging (PET scans)
Carbon Dating
Allows scientists to determine the age of ancient artifacts
Nuclear Energy
Part of nuclear reactions that generate electricity
Understanding beta decay helps scientists:
• Develop new medical treatments
• Study the history of our planet
• Generate clean energy
• Understand how stars create elements
While radiation requires safety precautions, beta decay has transformed modern science and medicine!
Beta Decay Quiz
Test your beta decay knowledge with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about beta decay:
Beta Decay Trivia
Discover some fascinating facts about beta decay:
Historical Detective
Carbon-14 dating relies on beta decay to determine the age of ancient artifacts. By measuring remaining carbon-14, scientists can date objects up to 50,000 years old!
Ghost Particles
Neutrinos emitted during beta decay are called "ghost particles" because they rarely interact with matter. Trillions pass through your body every second without effect!
Medical Marvel
Beta decay is used in cancer treatment. Radioactive isotopes like Strontium-90 emit beta particles that target and destroy cancer cells while sparing healthy tissue.
Stellar Alchemy
Beta decay plays a crucial role in stars! It helps transform elements during nuclear fusion, creating heavier elements from lighter ones over billions of years.





