Ideal Gas Law - Definition, Examples, Quiz, FAQ, Trivia
Discover how gases behave when temperature, pressure, and volume change!
What is the Ideal Gas Law?
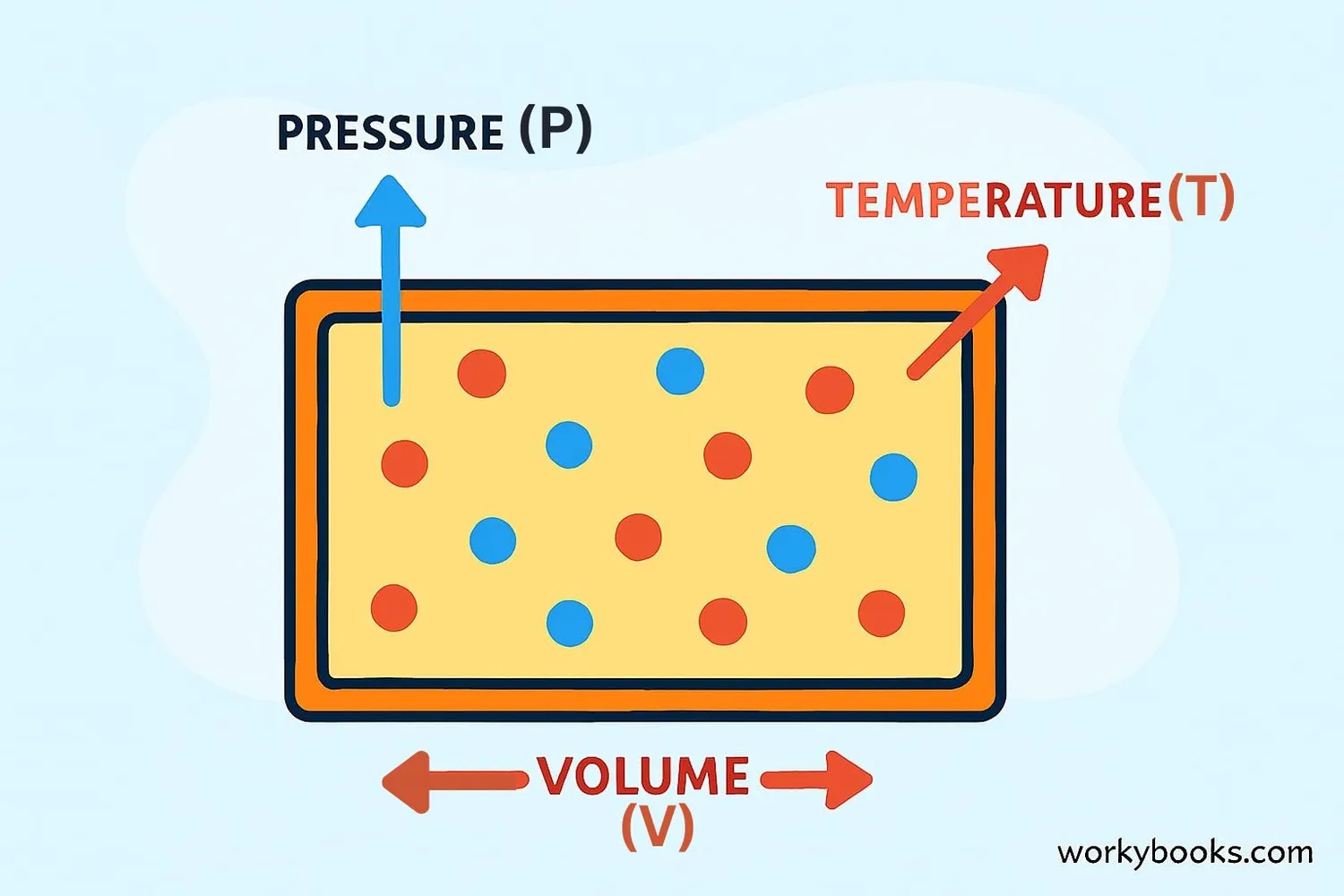
The Ideal Gas Law is a scientific rule that helps us understand how gases behave! It shows the relationship between four important things:
• Pressure (how much the gas pushes on its container)
• Volume (how much space the gas takes up)
• Temperature (how hot or cold the gas is)
• Amount (how many gas molecules there are)
Scientists use a special formula to describe this relationship:
Where:
• P = Pressure
• V = Volume
• n = Number of moles (amount of gas)
• R = Gas constant (a special number that never changes)
• T = Temperature (in Kelvin)
Science Fact!
An "ideal gas" is a theoretical gas that perfectly follows this law. Real gases come close to ideal behavior at high temperatures and low pressures.
How the Ideal Gas Law Works
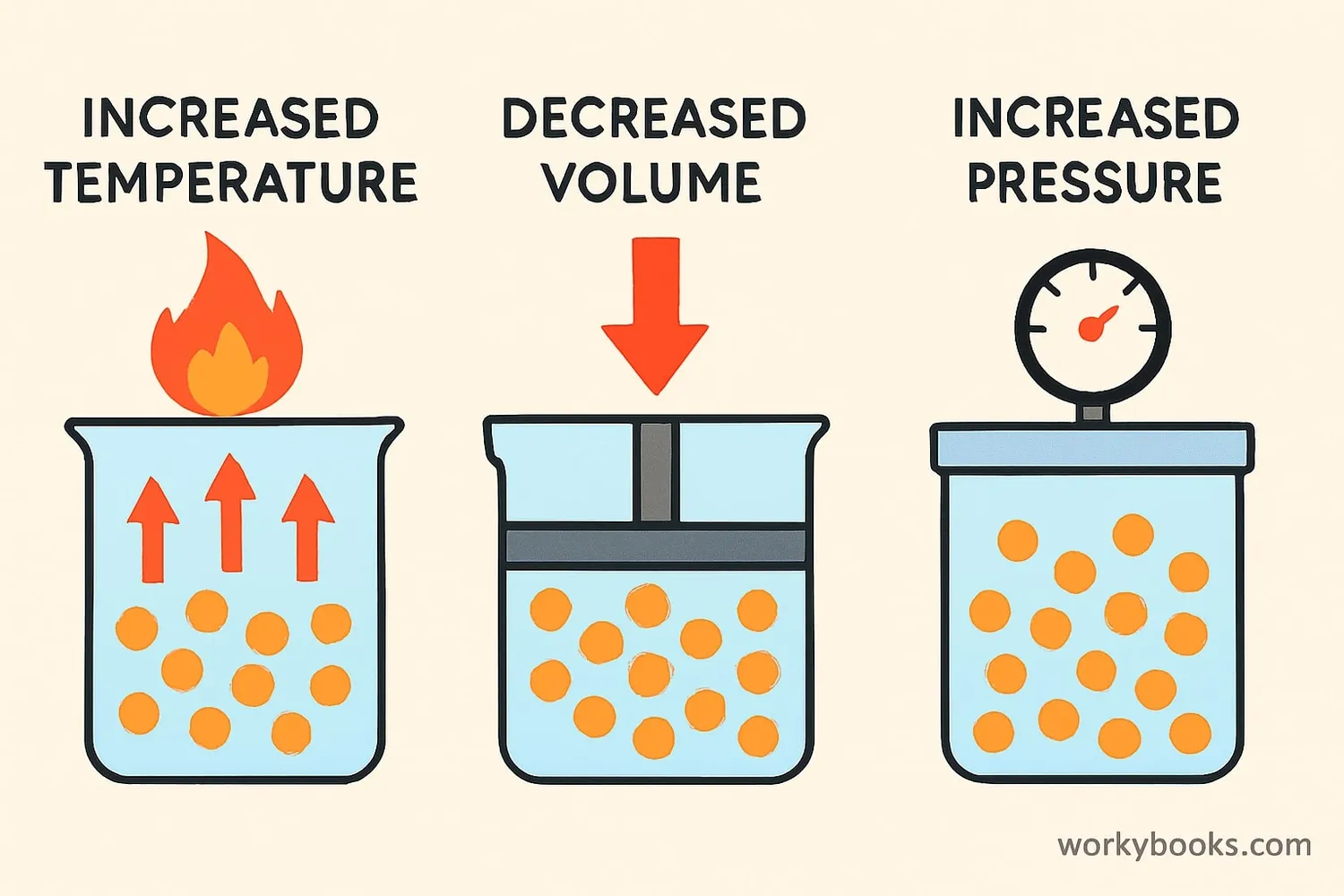
The Ideal Gas Law helps us predict what happens when we change one of the gas properties. Here are the key relationships:
Pressure & Volume
When temperature is constant, increasing pressure makes volume decrease (Boyle's Law)
Volume & Temperature
When pressure is constant, increasing temperature makes volume increase (Charles's Law)
Pressure & Temperature
When volume is constant, increasing temperature makes pressure increase (Gay-Lussac's Law)
Amount of Gas
Adding more gas molecules increases pressure or volume depending on conditions
Real World Example: When you inflate a bicycle tire, you're adding more gas molecules (increasing n). This increases the pressure inside the tire (P) because the volume (V) is mostly fixed by the tire's shape.
Temperature Matters!
The Ideal Gas Law uses Kelvin temperature instead of Celsius or Fahrenheit. To convert Celsius to Kelvin, just add 273!
Why the Ideal Gas Law is Important
The Ideal Gas Law isn't just for scientists! It helps us understand and design many things in our world:
Weather Forecasting
Helps predict how air masses will move and change with temperature
Car Engines
Explains how fuel and air mix and expand to power vehicles
Medical Devices
Helps design equipment like ventilators and anesthesia machines
Without understanding gas laws, we couldn't:
• Predict weather accurately
• Design efficient engines
• Create life-saving medical equipment
• Understand how our atmosphere works
• Develop refrigeration and air conditioning
The Ideal Gas Law helps scientists and engineers solve problems and create technologies that make our lives better!
Ideal Gas Law Quiz
Test your understanding of gas laws with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about the ideal gas law:
Fun Gas Law Trivia
Discover some amazing facts about gases and the ideal gas law!
Space Exploration
NASA uses the ideal gas law to design space suits! The law helps engineers calculate how much air astronauts need and how it will behave in the vacuum of space.
Deep Sea Diving
Scuba divers must understand gas laws. As they go deeper, pressure increases, which affects how gases dissolve in their blood - a critical safety consideration!
Historical Development
The ideal gas law wasn't discovered all at once! Different scientists discovered parts of it over 200 years, starting with Robert Boyle in 1662 and completed by Émile Clapeyron in 1834.
Cooking Science
When you bake bread, gas laws are at work! Yeast produces CO₂ gas that expands as the dough warms in the oven, making the bread rise and creating those fluffy air pockets.





