Kinetic Molecular Theory - Definition, Examples, Quiz, FAQ, Trivia
Discover how tiny particles create gas pressure and temperature!
What is Kinetic Molecular Theory?
Kinetic Molecular Theory (KMT) is a scientific model that helps us understand how gases behave. It explains that all gases are made of tiny particles that are constantly moving and colliding with each other and their container.
Think of gas particles like super tiny, super fast marbles bouncing around inside a box! The word "kinetic" means movement, and "molecular" refers to molecules, so KMT is all about how molecules move. This theory helps scientists explain why gases spread out to fill their containers, why they can be compressed, and how temperature affects them.
Science Fact!
At room temperature, gas molecules move at speeds of about 500 meters per second - that's faster than a jet plane!
The 5 Key Postulates
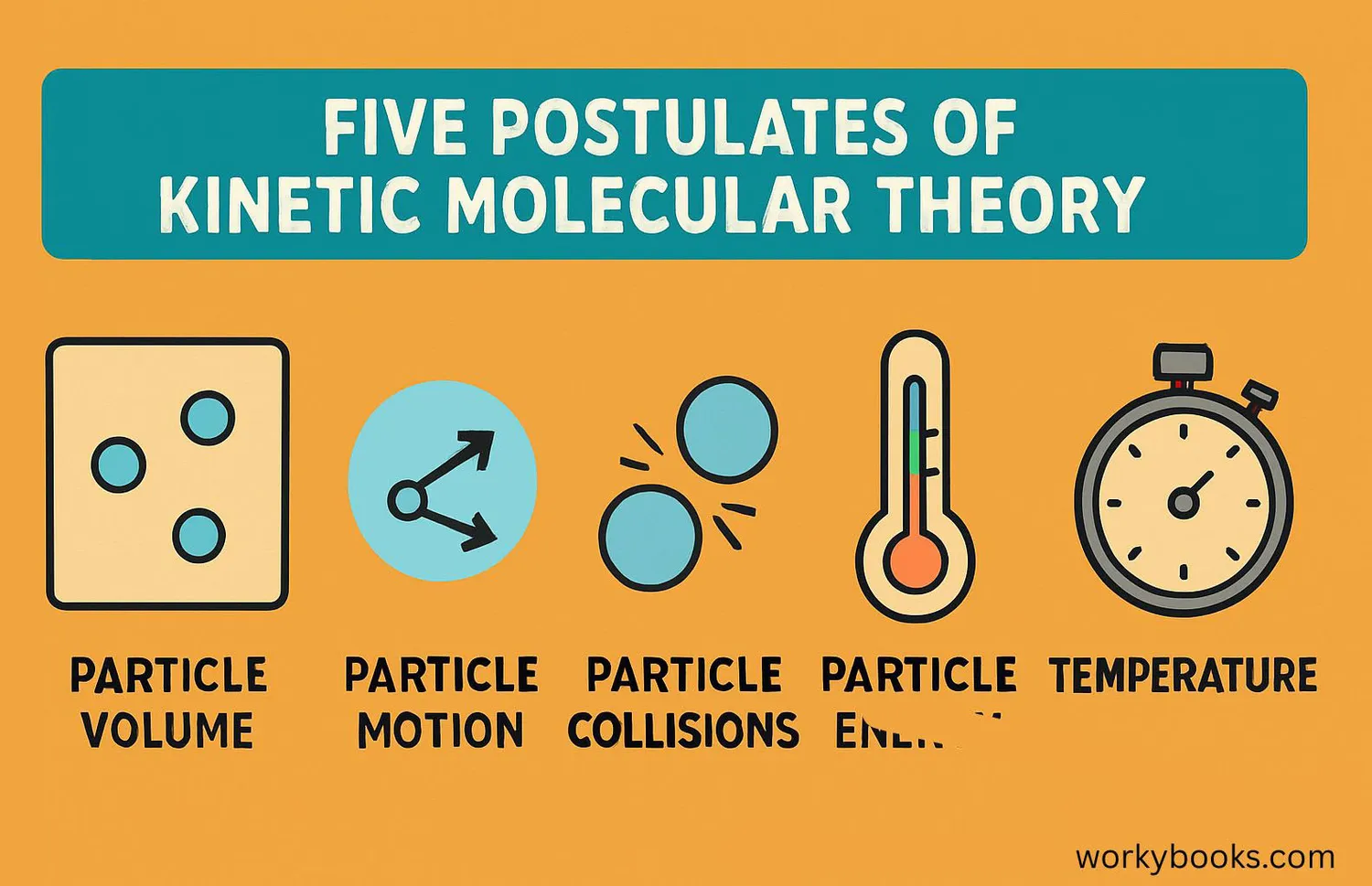
Scientists developed five main ideas (called postulates) that form the foundation of Kinetic Molecular Theory. These postulates help explain how gases behave:
Constant Motion
Gas particles are in constant, random, straight-line motion
Small Size
The particles are so small that their volume is negligible
No Attraction
Particles don't attract or repel each other
Elastic Collisions
Collisions between particles are perfectly elastic
Temperature = Energy
Temperature measures the average kinetic energy
These five ideas might seem simple, but they help explain everything from why balloons inflate to how refrigerators work. For example, postulate #4 means that when gas particles collide, they bounce off each other without losing energy - like perfect billiard balls!
Elastic Collision Fact!
In one second, a single gas molecule might collide with other molecules billions of times!
How KMT Explains Gas Laws
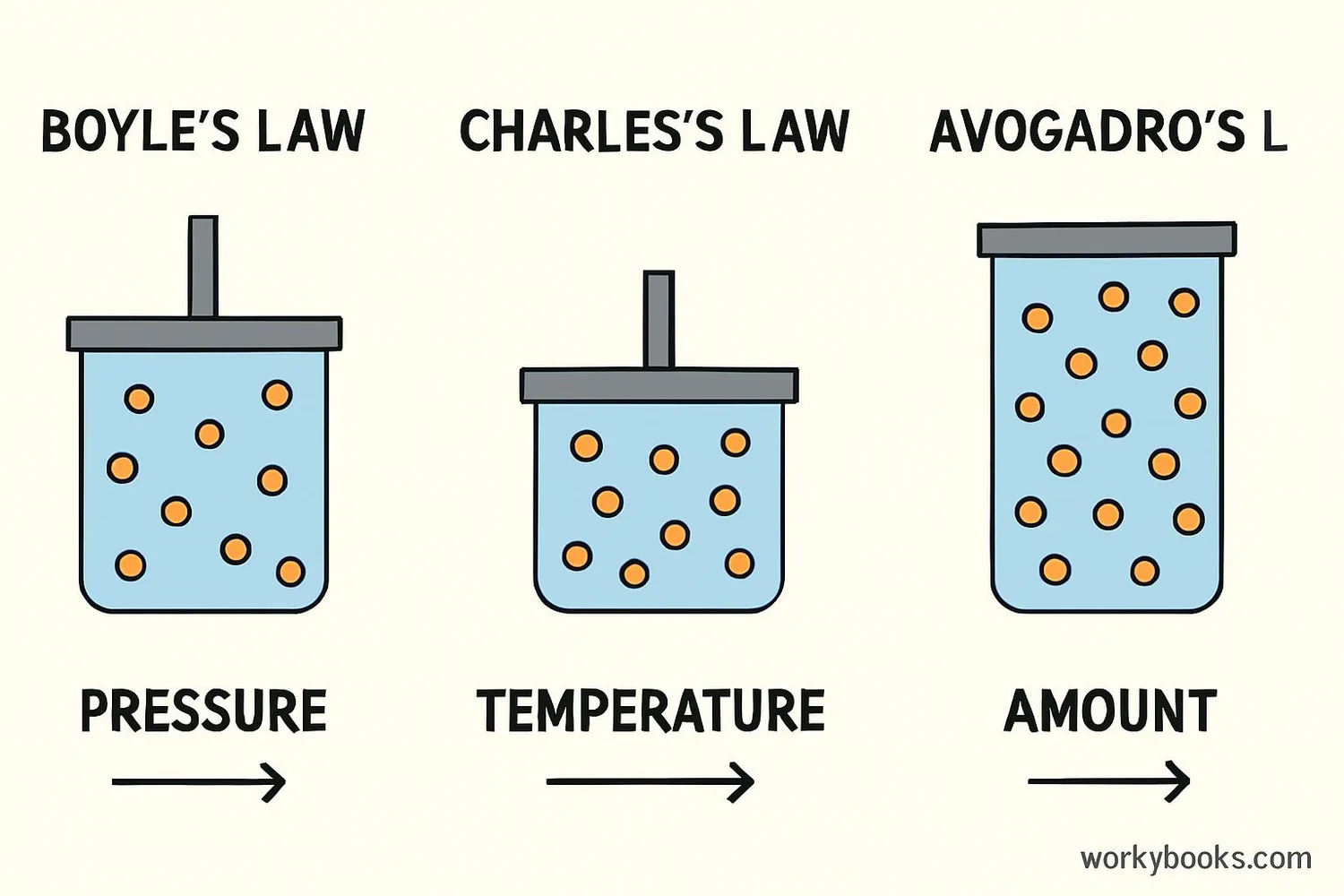
Kinetic Molecular Theory helps explain the gas laws that scientists have discovered through experiments. These laws describe how gases respond to changes in pressure, volume, temperature, and quantity:
Boyle's Law
Pressure and Volume: When you decrease a gas's volume (like pushing down on a syringe), the particles hit the container walls more often, increasing pressure.
Charles's Law
Volume and Temperature: When you heat a gas, particles move faster and push harder against the container, increasing volume if pressure stays constant.
Avogadro's Law
Volume and Quantity: Adding more gas particles to a container increases the number of collisions with the walls, increasing pressure unless volume expands.
These gas laws combine into the Ideal Gas Law: PV = nRT, where:
P = Pressure, V = Volume, n = number of particles, R = constant, T = Temperature
This equation helps scientists predict how gases will behave in different situations, from weather patterns to car engines!
Temperature & Kinetic Energy
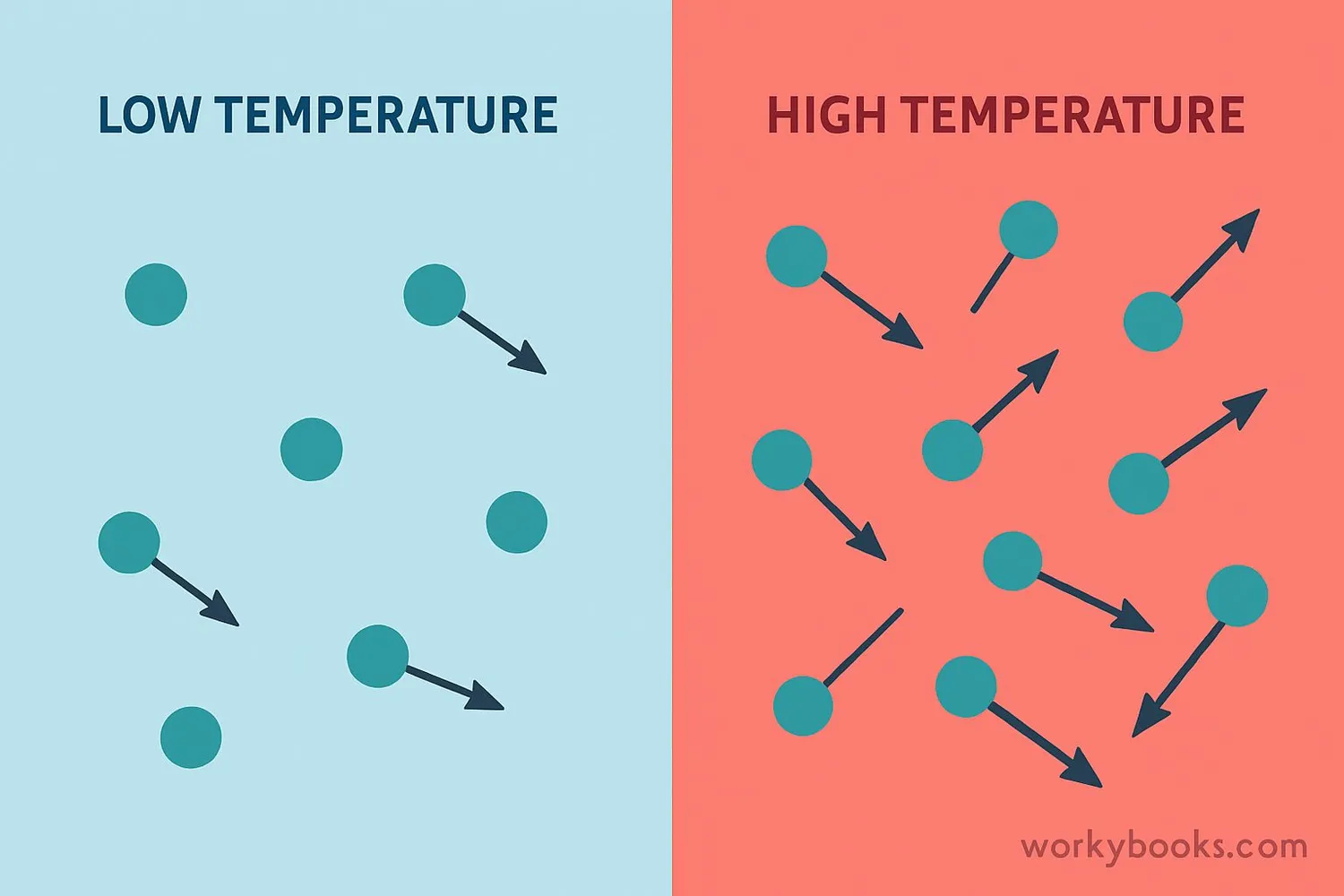
According to Kinetic Molecular Theory, temperature is directly related to the average kinetic energy of gas particles. This means:
• When you heat a gas, its particles move faster
• When you cool a gas, its particles move slower
The kinetic energy (energy of motion) of gas particles is given by the equation:
KE = ½mv²
Where m is mass and v is velocity (speed).
Cold Temperature
Particles move slowly with low kinetic energy
Hot Temperature
Particles move rapidly with high kinetic energy
Same Temperature
Different gases have same average kinetic energy
This relationship explains why hot air rises (faster-moving particles spread out more) and why pressure increases when you heat a sealed container (particles hit the walls harder and more often).
Kinetic Theory Quiz
Test your understanding with this quiz! Answer all 5 questions to see how much you've learned about Kinetic Molecular Theory.
Frequently Asked Questions
Here are answers to some common questions about Kinetic Molecular Theory:
Gas Science Trivia
Discover some amazing facts about gases and molecular motion!
Space Gases
In the near-vacuum of space, gas molecules are so spread out that they might travel kilometers before colliding with another molecule!
Speed of Molecules
Hydrogen molecules at room temperature move at about 1,800 meters per second - that's faster than a bullet!
Historical Discovery
The Kinetic Molecular Theory was developed in the 19th century by scientists including James Clerk Maxwell and Ludwig Boltzmann.
Absolute Zero
At absolute zero (-273°C), molecular motion theoretically stops completely. Scientists have gotten within a billionth of a degree of this temperature!





