Methane Hydrates - Definition, Examples, Quiz, FAQ, Trivia
Discover how frozen natural gas beneath the ocean floor powers our planet!
What Are Methane Hydrates?
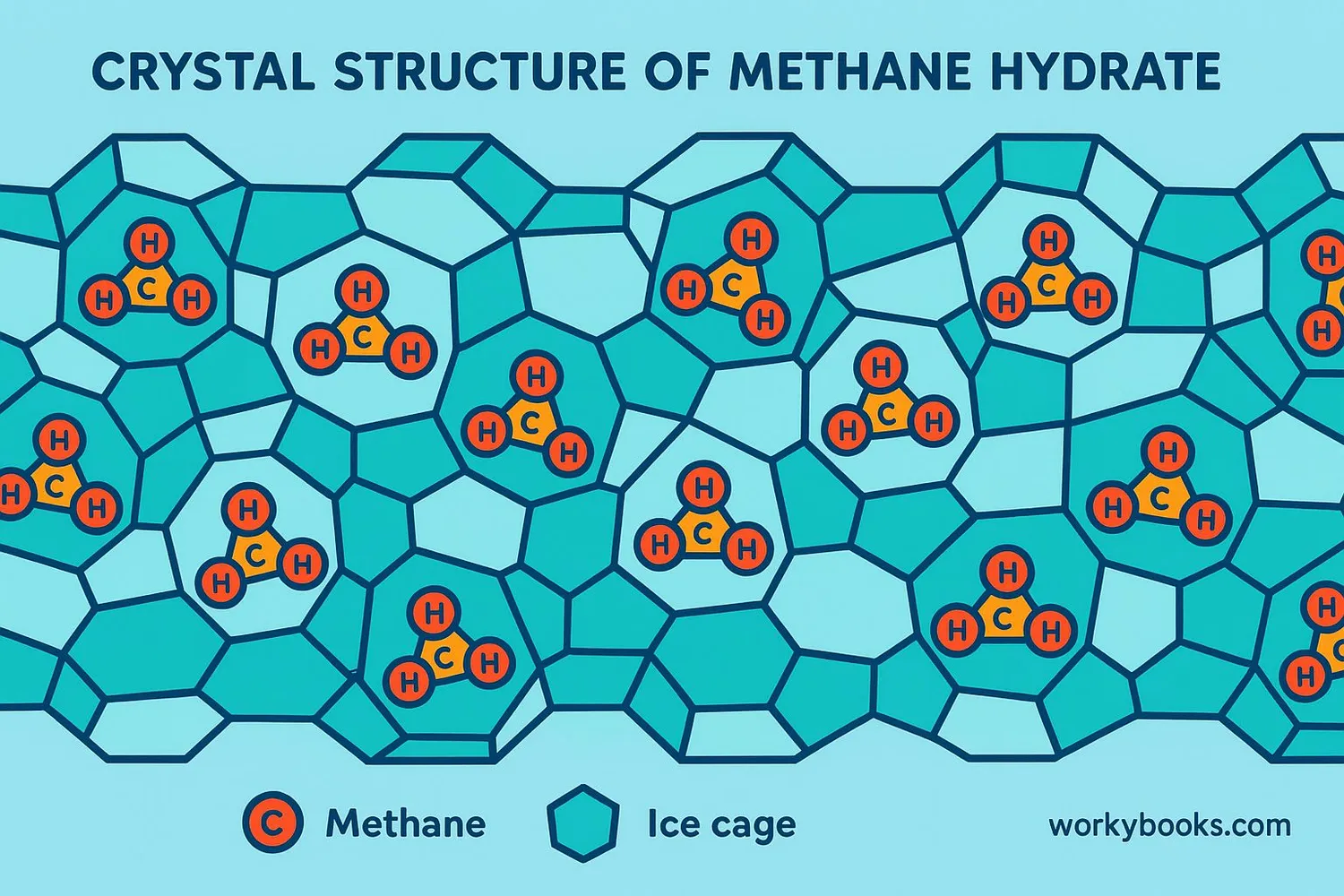
Methane hydrates are like frozen energy! They're special ice crystals that trap natural gas (methane) inside their structure. Imagine a cage made of ice with a methane molecule trapped inside - that's a methane hydrate!
Scientists call them "clathrate hydrates" or "natural gas hydrates." They form under very specific conditions - high pressure and low temperature - which is why we find them deep in the ocean or beneath Arctic permafrost.
Cool Fact!
Methane hydrates can actually burn! If you light a piece of methane hydrate ice, it will burn with a flame as the methane gas escapes.
How Methane Hydrates Form
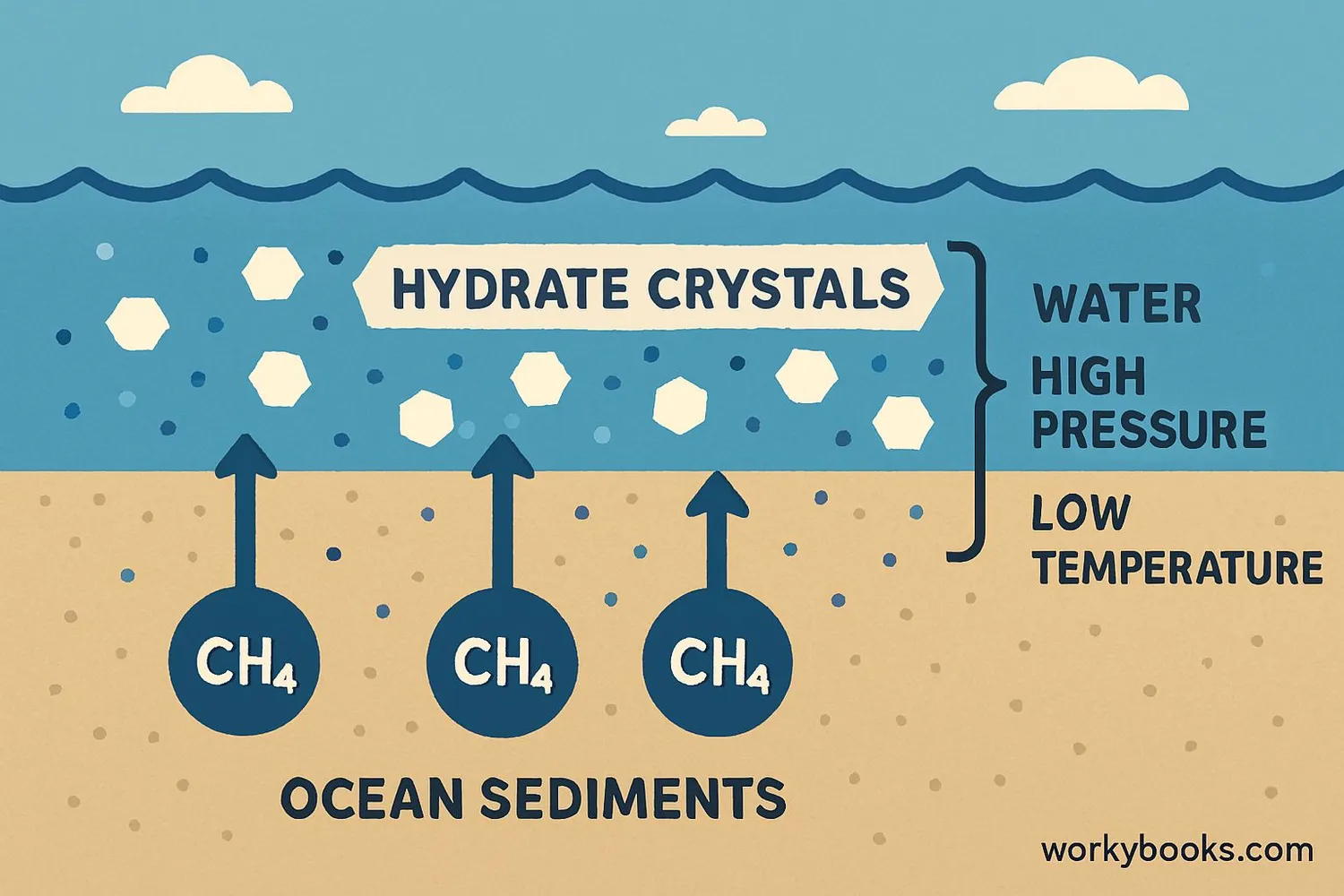
Methane hydrates form through a fascinating natural process:
Methane Production
Microorganisms break down organic matter in ocean sediments, producing methane gas
Deep Ocean Conditions
Water depth creates high pressure and cold temperatures
Hydrate Formation
Methane combines with water to form ice-like crystals
Stable Storage
Hydrates remain stable under ocean floor conditions
This special combination of methane gas, water, cold temperatures, and high pressure creates the perfect conditions for methane hydrates to form. They're most stable at ocean depths greater than 300 meters where the water is very cold.
Ocean Chemistry!
One cubic meter of methane hydrate contains about 164 cubic meters of methane gas! That's why they're such an energy-rich resource.
Where Methane Hydrates Are Found
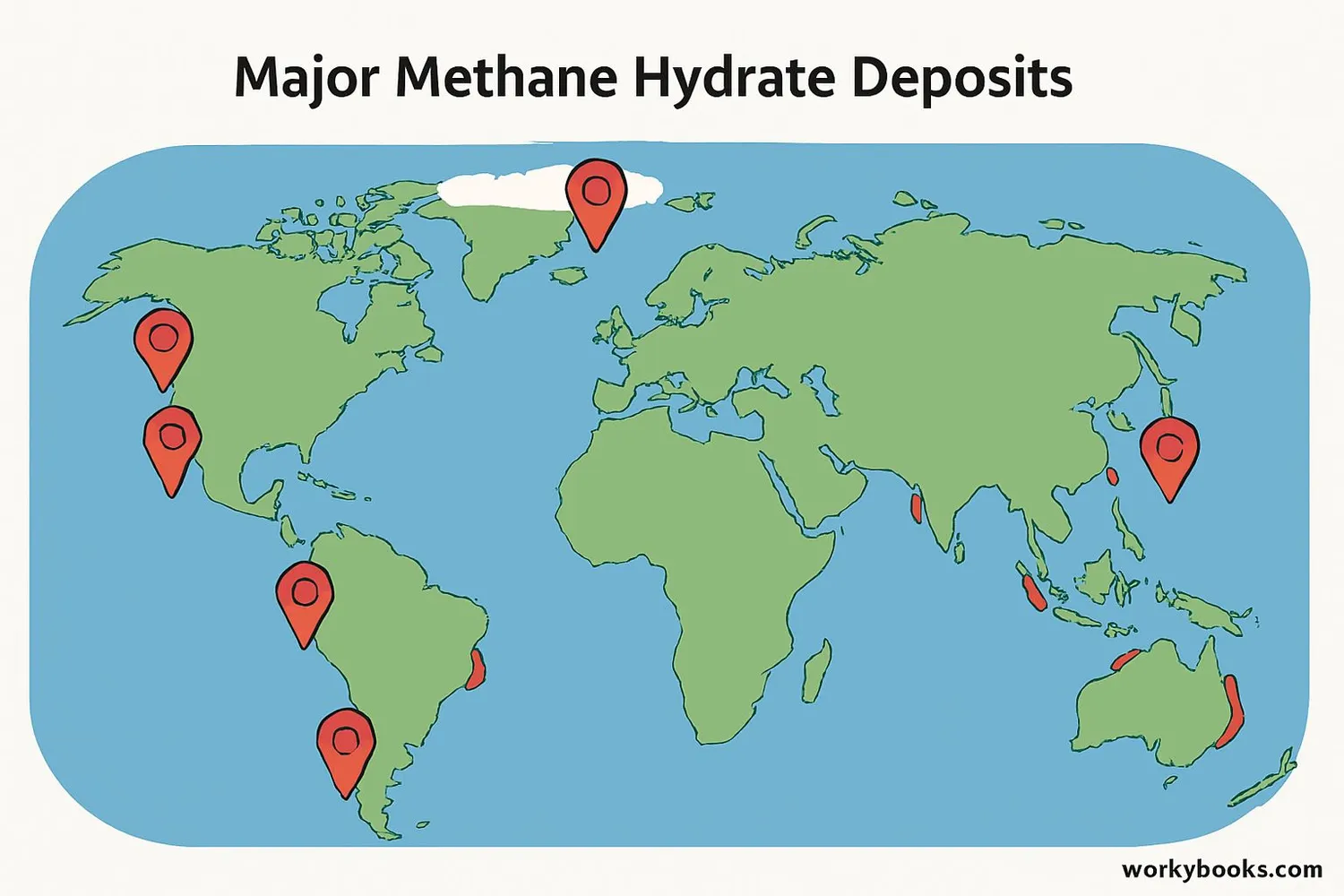
Methane hydrates are found all around the world in special ocean environments:
Continental Margins
Along the edges of continents where the ocean meets land
Deep Ocean Sediments
Buried under the seafloor where pressure is high
Arctic Permafrost
Beneath frozen ground in polar regions
Major deposits have been found off the coasts of Japan, India, the United States, Canada, and in the Arctic Ocean. Scientists estimate that there might be more energy stored in methane hydrates than in all other fossil fuels combined!
Why Methane Hydrates Are Important

Methane hydrates are important for two big reasons:
1. Energy Resource: They contain enormous amounts of natural gas that could potentially power our homes, schools, and cities for centuries. Scientists are researching ways to safely extract this energy.
2. Climate Change: Methane is a powerful greenhouse gas. If ocean temperatures rise, methane hydrates could melt and release methane into the atmosphere, accelerating global warming.
Energy Potential!
Some estimates suggest there's twice as much carbon in methane hydrates as in all other fossil fuels combined!
Methane Hydrates Quiz
Test your knowledge about methane hydrates with this interactive quiz!
Frequently Asked Questions
Here are answers to common questions about methane hydrates:
Fun Methane Hydrate Trivia
Discover amazing facts about methane hydrates!
Burning Ice
Methane hydrates are sometimes called "fire ice" because you can literally set them on fire! The ice melts and releases methane gas that burns with a flame.
Massive Reserves
There's more energy in methane hydrates than in all other fossil fuels combined! The U.S. Geological Survey estimates there may be 700,000 trillion cubic feet worldwide.
Not Just on Earth
Methane hydrates exist on other planets too! Scientists have found evidence of them on Mars and on moons like Titan, where they might form in similar cold conditions.
Ancient Climate Events
Scientists think massive methane hydrate releases may have caused sudden global warming events in Earth's past, like the Paleocene-Eocene Thermal Maximum 55 million years ago.





