Crystal Science - Definition, Examples, Quiz, FAQ, Trivia
Discover how crystals form, their structure, and amazing properties
What are Crystals?

Crystals are solid materials where the atoms are arranged in a repeating pattern that extends in all three spatial dimensions. This special arrangement gives crystals their unique shapes and properties.
Think of crystals as nature's LEGO blocks! Just like how LEGO pieces fit together in patterns, atoms in crystals connect in regular, repeating ways. This organized structure is what makes crystals different from other solids.
Crystal Fact!
Crystals can form naturally or be made in laboratories. Snowflakes, salt, and diamonds are all crystals!
Crystal Structure and Lattice
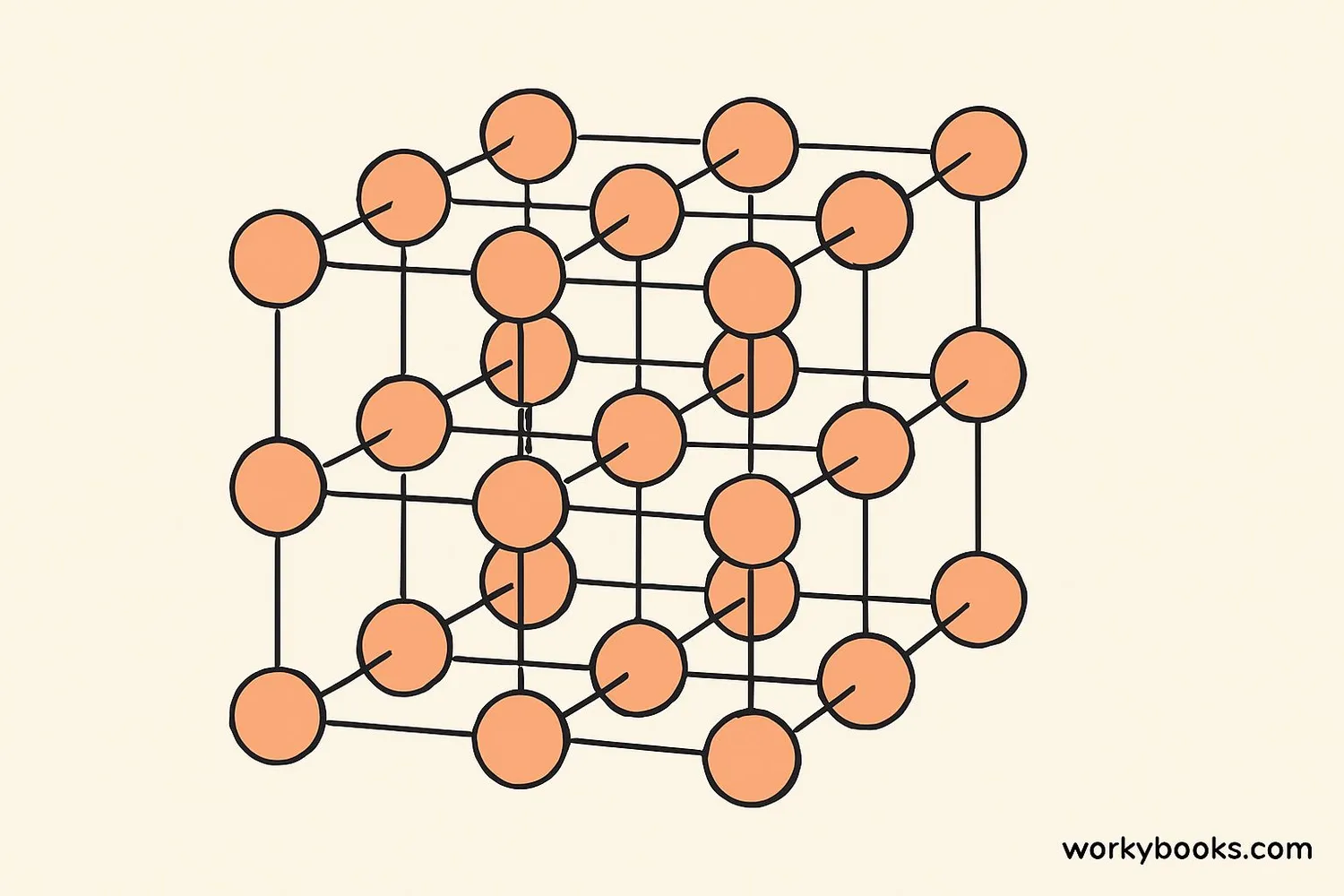
The secret behind every crystal is its crystal lattice - the organized framework that holds the atoms in place. This lattice is like a 3D grid where each point represents an atom or molecule.
Unit Cell
The smallest repeating unit in a crystal structure
Lattice Points
Positions where atoms or molecules are located
Crystal Systems
Seven basic geometric arrangements
There are seven main crystal systems that describe how the unit cells are arranged:
Cubic, Tetragonal, Orthorhombic, Monoclinic, Triclinic, Trigonal, and Hexagonal
Each system has unique angles and side lengths that give crystals their distinctive shapes.
Symmetry Matters!
The angles between crystal faces are always the same for a particular mineral, no matter the size of the crystal!
How Crystals Form
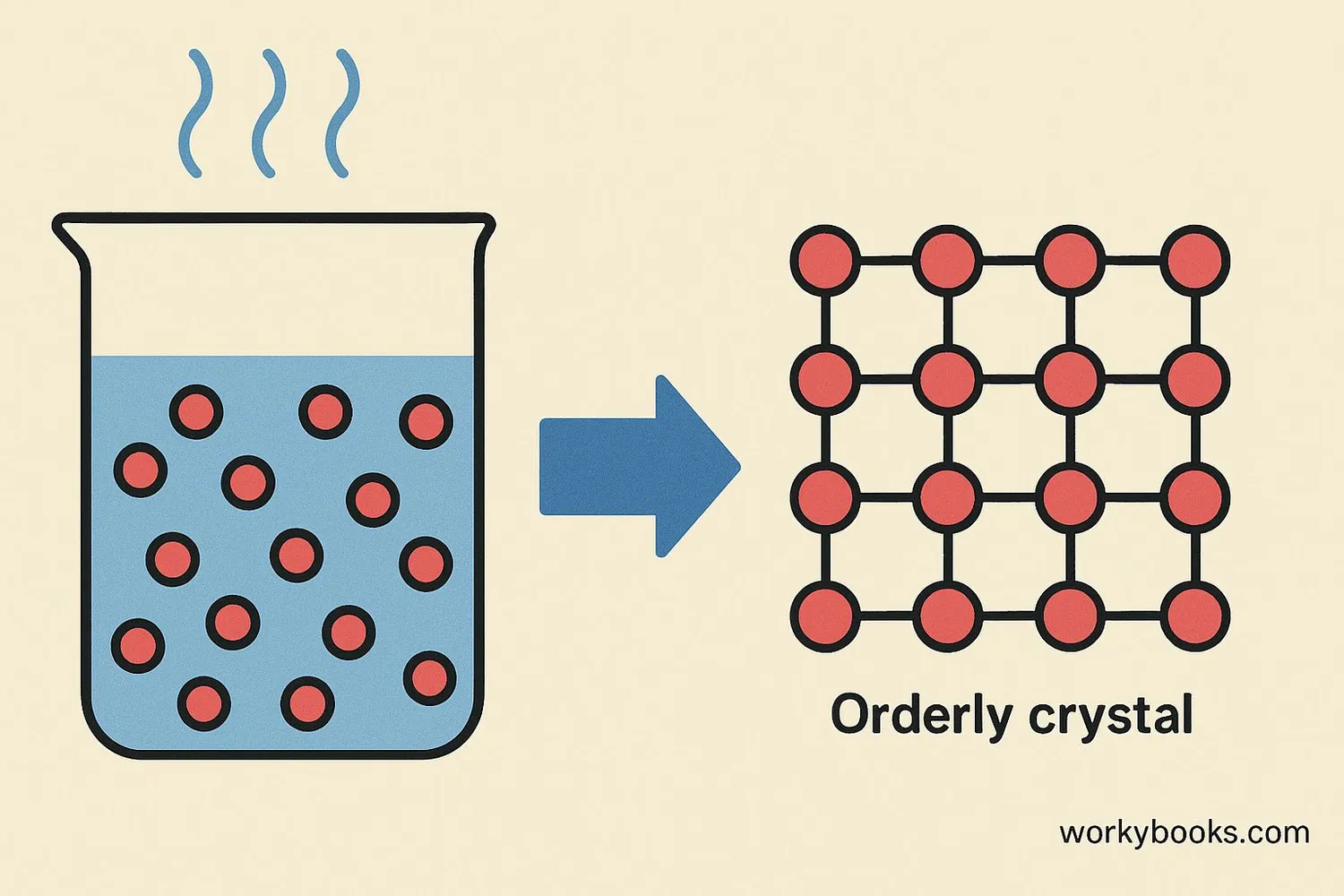
Crystals form through a process called crystallization. This happens when atoms or molecules arrange themselves into a highly organized structure. There are several ways this can occur:
Cooling
Molten rock (magma) cools slowly, allowing crystals to form
Evaporation
Water evaporates from a solution, leaving crystals behind
Pressure
Extreme pressure rearranges atoms into crystal structures
The size of crystals depends on the formation conditions:
• Slow cooling = Large crystals
• Fast cooling = Small crystals
• Pure solutions = Clear, well-formed crystals
• Impure solutions = Irregular crystals
You can grow your own crystals at home by dissolving salt or sugar in hot water and letting it cool slowly!
Types of Crystals
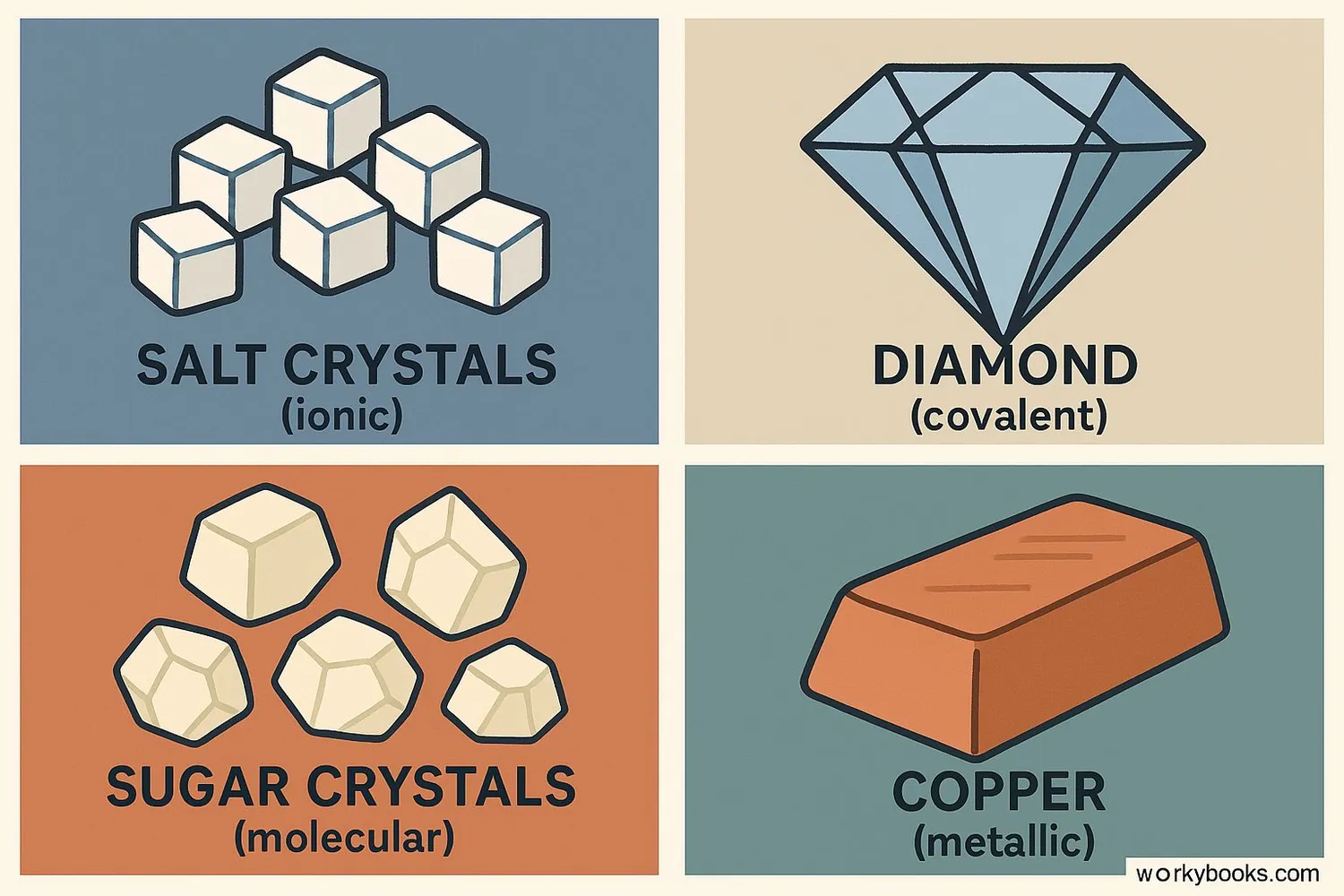
Crystals are classified based on the type of bonds holding their atoms together:
Ionic Crystals
Formed by ionic bonds (e.g., salt)
Properties: Hard, brittle, high melting points
Covalent Crystals
Formed by covalent bonds (e.g., diamond)
Properties: Very hard, high melting points
Molecular Crystals
Held by intermolecular forces (e.g., sugar)
Properties: Soft, low melting points
Metallic Crystals
Metal atoms in electron sea (e.g., copper)
Properties: Malleable, good conductors
Each type has unique properties based on their bonding:
• Ionic crystals conduct electricity when dissolved
• Covalent crystals are often gemstones
• Molecular crystals dissolve easily in water
• Metallic crystals are shiny and bendable
Crystal Knowledge Quiz
Test your crystal knowledge with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about crystals:
Crystal Trivia
Discover fascinating facts about crystals:
Giant Crystals
The largest natural crystals on Earth are in Mexico's Cave of Crystals. Some selenite crystals are over 36 feet long and weigh 55 tons!
Space Crystals
Scientists have discovered a star that's essentially a giant diamond crystal! It's a white dwarf star about 4,000 km across made of crystallized carbon.
Ancient Timekeepers
Quartz crystals in watches keep time by vibrating at a precise frequency when electricity passes through them - 32,768 times per second!
Liquid Crystals
Some materials can be liquid and crystalline at the same time! These "liquid crystals" flow like liquids but have molecules arranged like crystals, used in LCD screens.





