Bromine Valence Electrons - Definition, Examples, Quiz, FAQ, Trivia
Discover how atoms share electrons and form bonds
What are Valence Electrons?
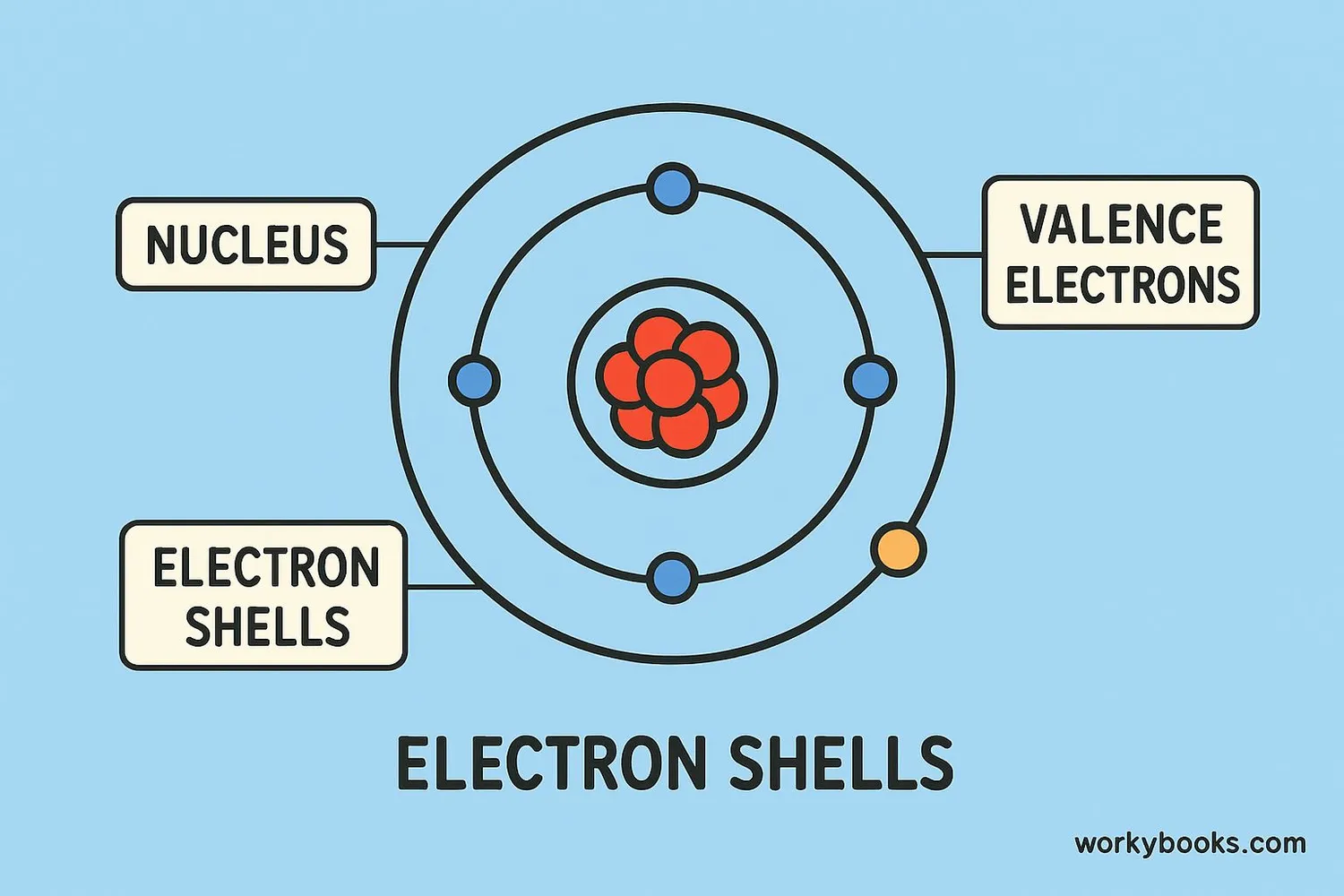
Valence electrons are the electrons in the outermost shell of an atom. They are special because they determine how atoms interact with each other and form chemical bonds.
Think of valence electrons like the hands of an atom - they're what atoms use to hold hands (form bonds) with other atoms. The number of valence electrons an atom has determines how many bonds it can make.
Key Fact!
The periodic table is organized so that elements in the same column (group) have the same number of valence electrons!
Bromine: The Element
Bromine is a chemical element with the symbol Br and atomic number 35. It's one of only two elements that are liquid at room temperature (the other is mercury). Bromine is part of the halogen group in the periodic table.
Bromine has a reddish-brown color and a strong odor. It's found in seawater and salt lakes, and it's used in flame retardants, photography chemicals, and some medicines.
Atomic Number
35 - Bromine has 35 protons
Group Number
Group 17 - Halogens
State at Room Temp
Liquid (reddish-brown)
Bromine's Electron Configuration
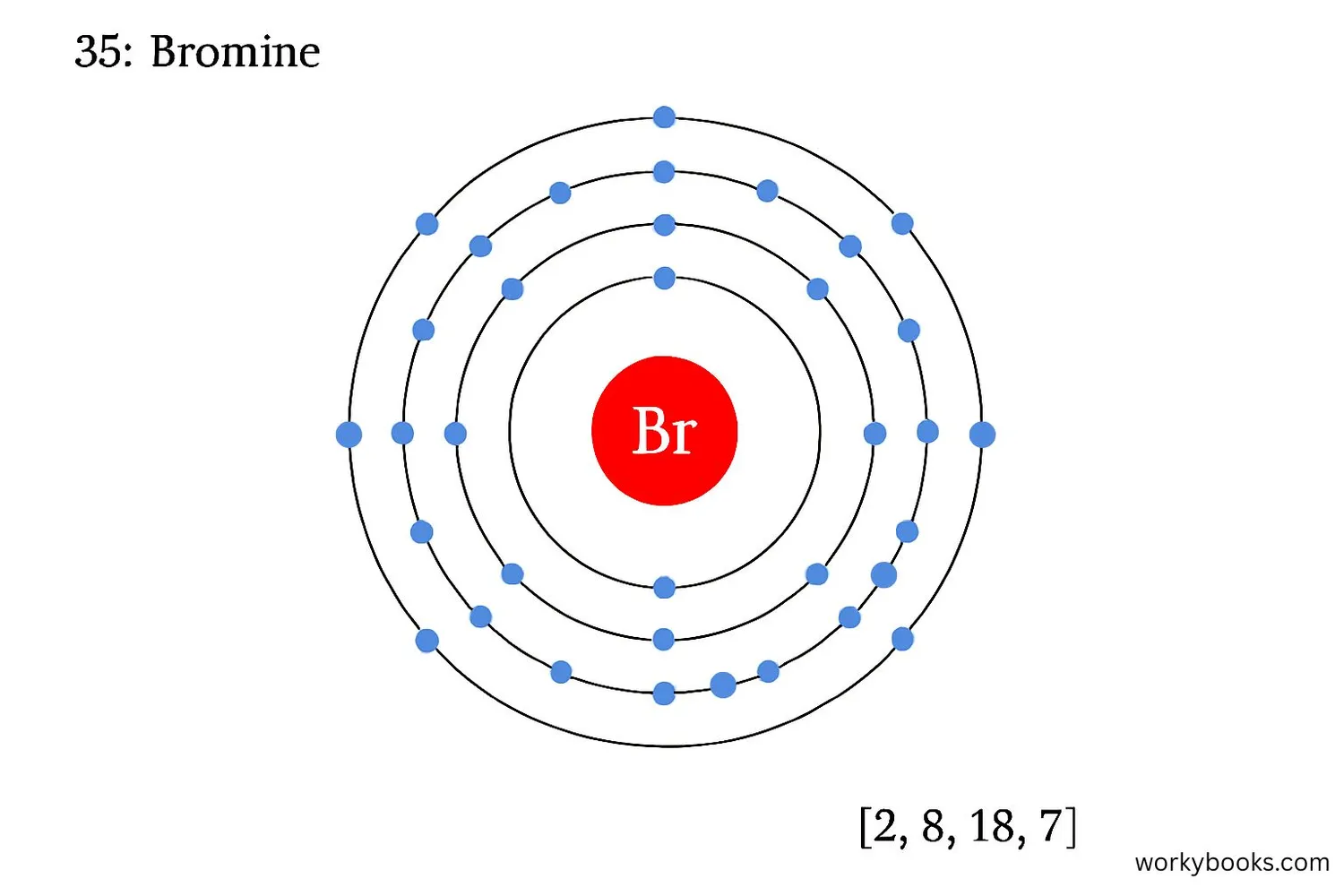
Electron configuration describes how electrons are arranged around an atom's nucleus. For bromine (atomic number 35), the electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
This means bromine has:
• 2 electrons in the first shell
• 8 electrons in the second shell
• 18 electrons in the third shell
• 7 electrons in the fourth shell
Valence Electrons Count
Bromine has 7 valence electrons because it has 7 electrons in its outermost shell (the fourth shell).
Bromine Lewis Dot Structure
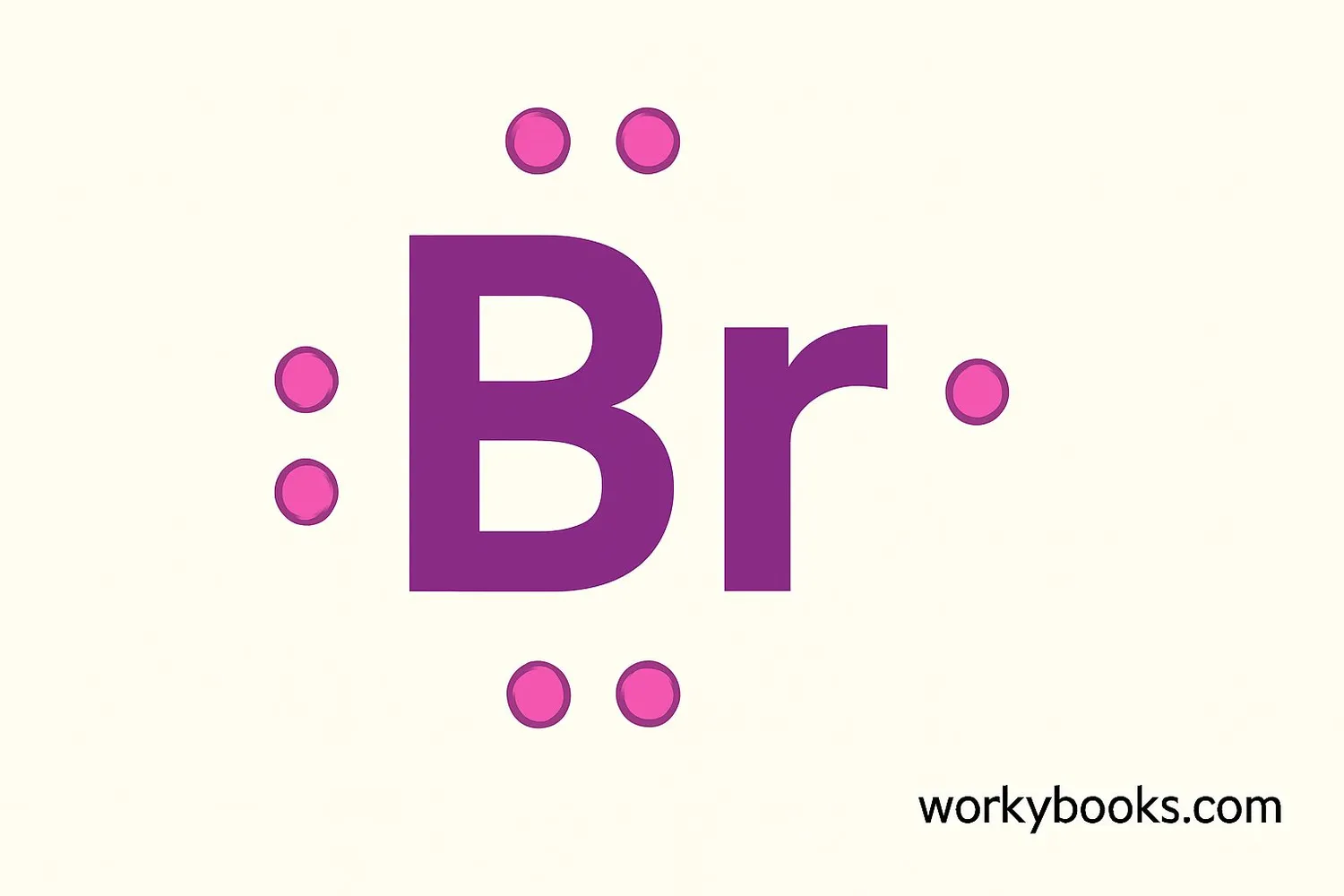
A Lewis dot structure is a simple way to show an atom's valence electrons. For bromine, we represent its 7 valence electrons as dots around the chemical symbol Br.
Here's how to draw bromine's Lewis dot structure:
1. Write the chemical symbol "Br"
2. Imagine a square around the symbol
3. Place one dot on each side of the square (top, right, bottom, left)
4. Place a second dot on three of the sides
5. One side will have a pair of dots, the other three sides will have single dots
This shows bromine has 7 valence electrons - one electron short of a full outer shell. That's why bromine readily forms bonds to gain that one extra electron!
Chemical Bonding
Bromine typically forms one bond because it needs just one more electron to complete its outer shell.
Bromine Valence Electrons Quiz
Test your knowledge about bromine and valence electrons with this quiz!
Frequently Asked Questions
Here are answers to common questions about bromine and valence electrons:
Fun Bromine Trivia
Discover some amazing facts about bromine and valence electrons!
Liquid Element
Bromine is one of only two elements that are liquid at room temperature! The other is mercury. Bromine evaporates easily, forming a reddish-brown vapor.
Name Origin
Bromine gets its name from the Greek word "bromos," meaning stench, because of its strong and unpleasant smell. It was discovered in 1826 by Antoine Balard.
Flame Retardant
Bromine compounds are excellent flame retardants. They're used in furniture, electronics, and textiles to prevent fires. The bromine atoms interfere with the chemical reactions that cause flames.
Photography Pioneer
Before digital cameras, bromine was essential for photography! Silver bromide was used in photographic film because it darkens when exposed to light, creating images.





