Chemical Properties of Matter - Definition, Examples, Quiz, FAQ, Trivia
Discover how matter changes and interacts through chemical properties
What are Chemical Properties?
Chemical properties describe how a substance changes into a different substance through chemical reactions. Unlike physical properties (like color or texture) that you can observe without changing the material, chemical properties show how matter behaves when it interacts with other matter.
Think of it this way: if you tear a piece of paper, you're changing its physical property (size and shape). But if you burn that paper, you're changing its chemical properties - it becomes ash and gases, which are completely different substances!
Science Fact!
Chemical properties are all about potential - they describe what a substance can do when it meets other substances, not what it looks like right now.
Types of Chemical Properties
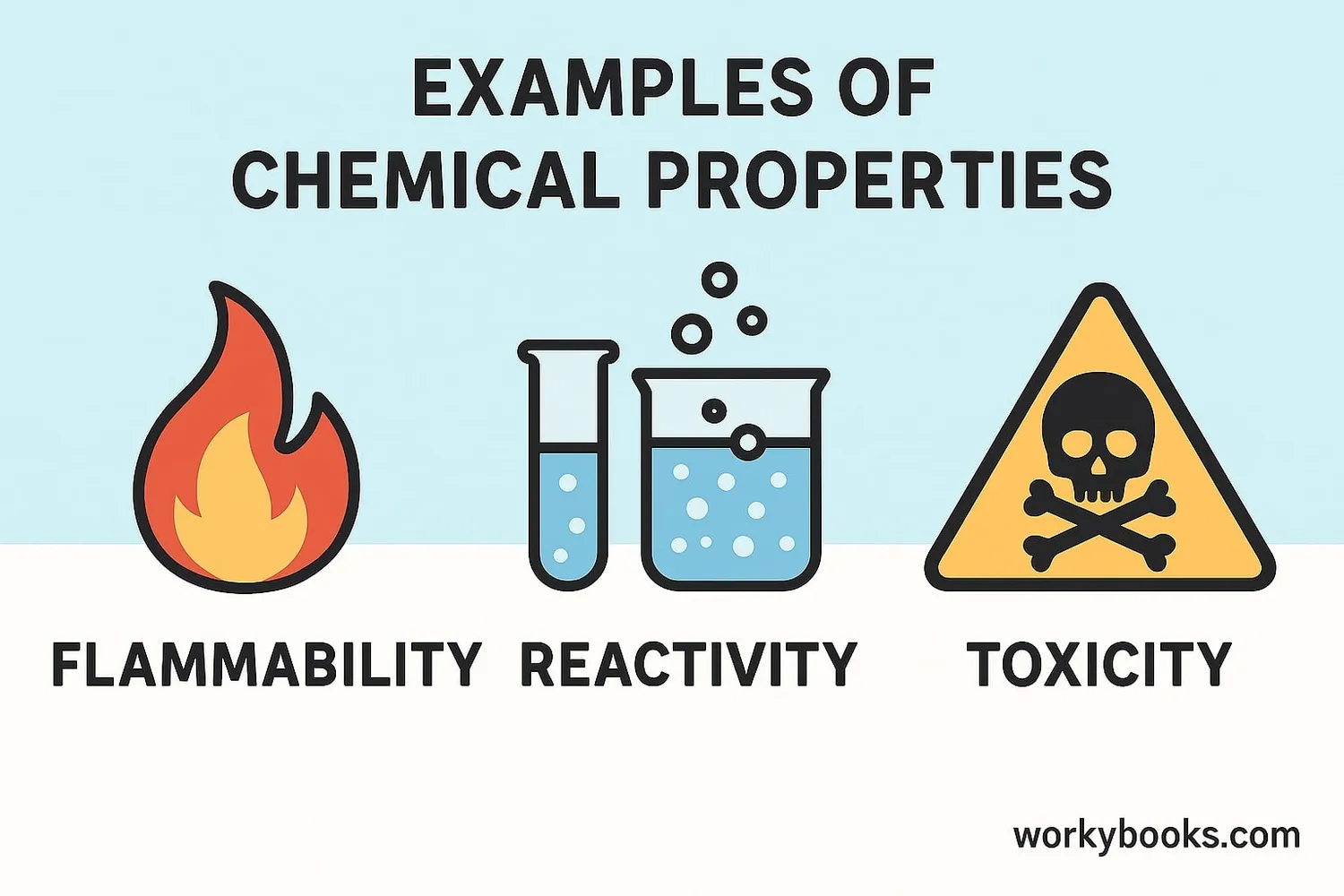
There are many different chemical properties that scientists study. Here are some of the most important ones:
Flammability
How easily a substance can catch fire and burn
Reactivity
How readily a substance combines chemically with other substances
Toxicity
How harmful a substance is to living organisms
Heat of Combustion
The amount of energy released when a substance burns completely
Chemical Stability
How resistant a substance is to undergoing chemical change
Acidity/Basicity
Whether a substance is an acid or a base (pH level)
Chemical properties are determined by the arrangement of atoms and the chemical bonds between them. When these bonds break and form new ones, we get a chemical change with different properties!
Chemical Bonds
Chemical bonds are like tiny hands that hold atoms together. Different types of bonds (ionic, covalent, metallic) give substances different chemical properties.
Why Chemical Properties Are Important

Understanding chemical properties helps us in many ways in our daily lives and in science:
Safety
Knowing toxicity and flammability helps us handle materials safely
Manufacturing
Chemical properties help create useful materials like plastics, medicines, and fuels
Environmental Protection
Understanding how substances interact helps prevent pollution
Chemical properties help explain:
• Why some materials corrode (like iron rusting)
• How batteries produce electricity
• Why baking soda makes cakes rise
• How digestion breaks down food
• Why we shouldn't mix certain cleaning products
By understanding chemical properties, scientists can create new materials with exactly the right characteristics for specific jobs!
Chemical Properties Quiz
Test your knowledge about chemical properties with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about chemical properties:
Fun Chemical Properties Trivia
Discover some amazing facts about chemical properties!
Golden Stability
Gold is prized for its chemical stability - it doesn't rust, tarnish, or react with most substances. This is why ancient gold artifacts still look shiny today!
Most Reactive Element
Fluorine is the most reactive chemical element. It's so reactive that it can make seemingly inert substances like glass, water, and even gold burn!
Special Water Properties
Water's chemical properties are unique - it expands when it freezes (most substances contract), and it can dissolve more substances than any other liquid.
Diamond and Graphite
Diamond and graphite are both made of carbon atoms, but their different atomic arrangements give them completely different chemical and physical properties!





