Electrons - Definition, Examples, Quiz, FAQ, Trivia
Discover the tiny particles that power our world!
What are Electrons?
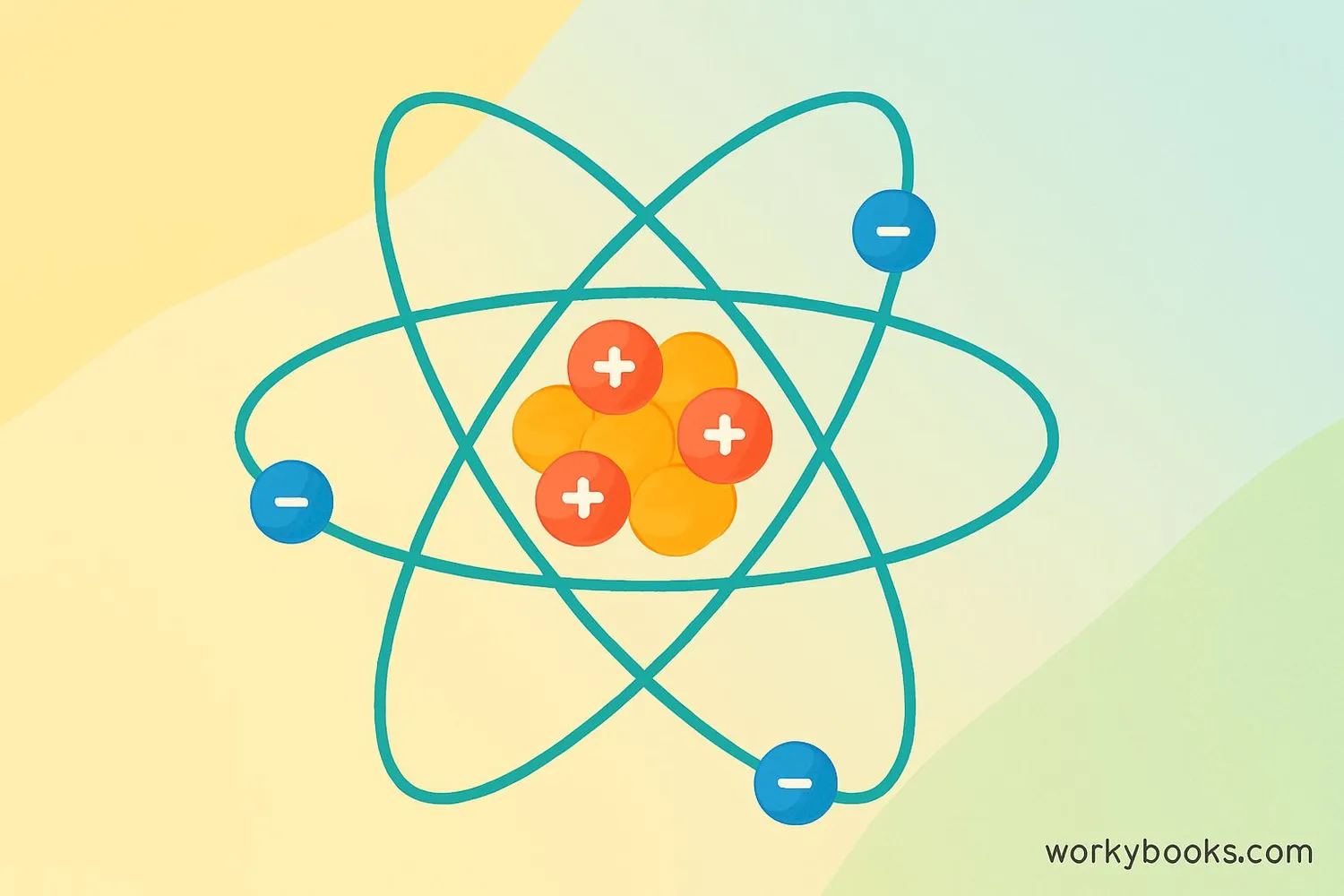
Electrons are tiny, negatively charged particles that are fundamental building blocks of all atoms. They're so small that it would take about 1,800 electrons to equal the mass of a single proton!
Imagine electrons as incredibly fast bees buzzing around a hive (the nucleus). They're constantly moving and can jump between different energy levels. Electrons play a crucial role in electricity, chemistry, and almost everything around us.
Discovery Fact!
Electrons were discovered by J.J. Thomson in 1897 through experiments with cathode rays. He noticed rays bending toward positive plates, revealing negative particles!
Atomic Structure
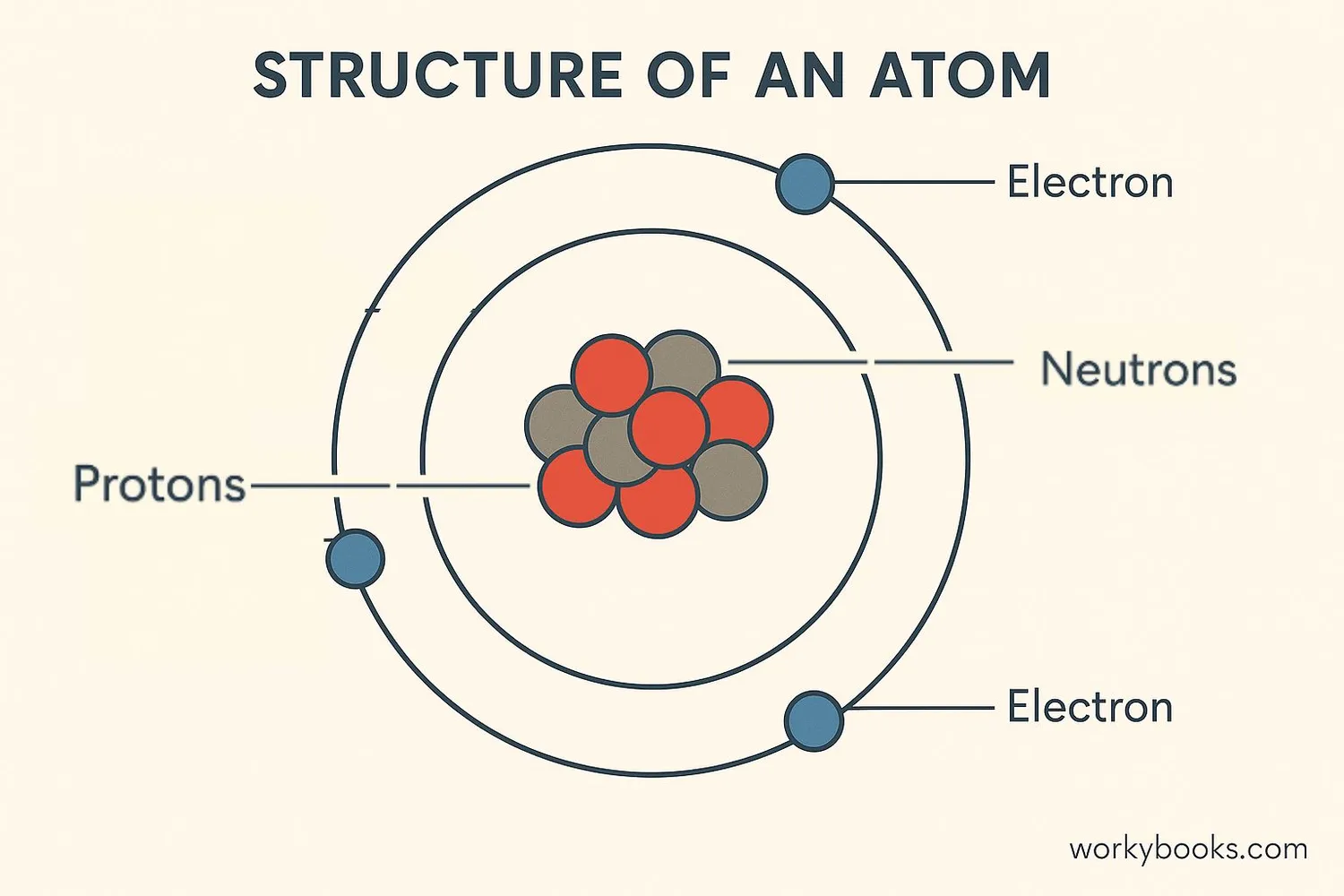
Every atom is made of three main particles:
Protons
Positively charged particles in the nucleus
Neutrons
Neutral particles in the nucleus
Electrons
Negatively charged particles orbiting the nucleus
Electrons live in specific regions called electron shells that surround the nucleus. The first shell holds up to 2 electrons, the second up to 8, and so on. The outermost electrons are called valence electrons and determine how atoms bond with each other.
Size Comparison
If an atom were the size of a football stadium, the nucleus would be a marble on the 50-yard line, and electrons would be like tiny gnats buzzing around the stands!
Properties of Electrons
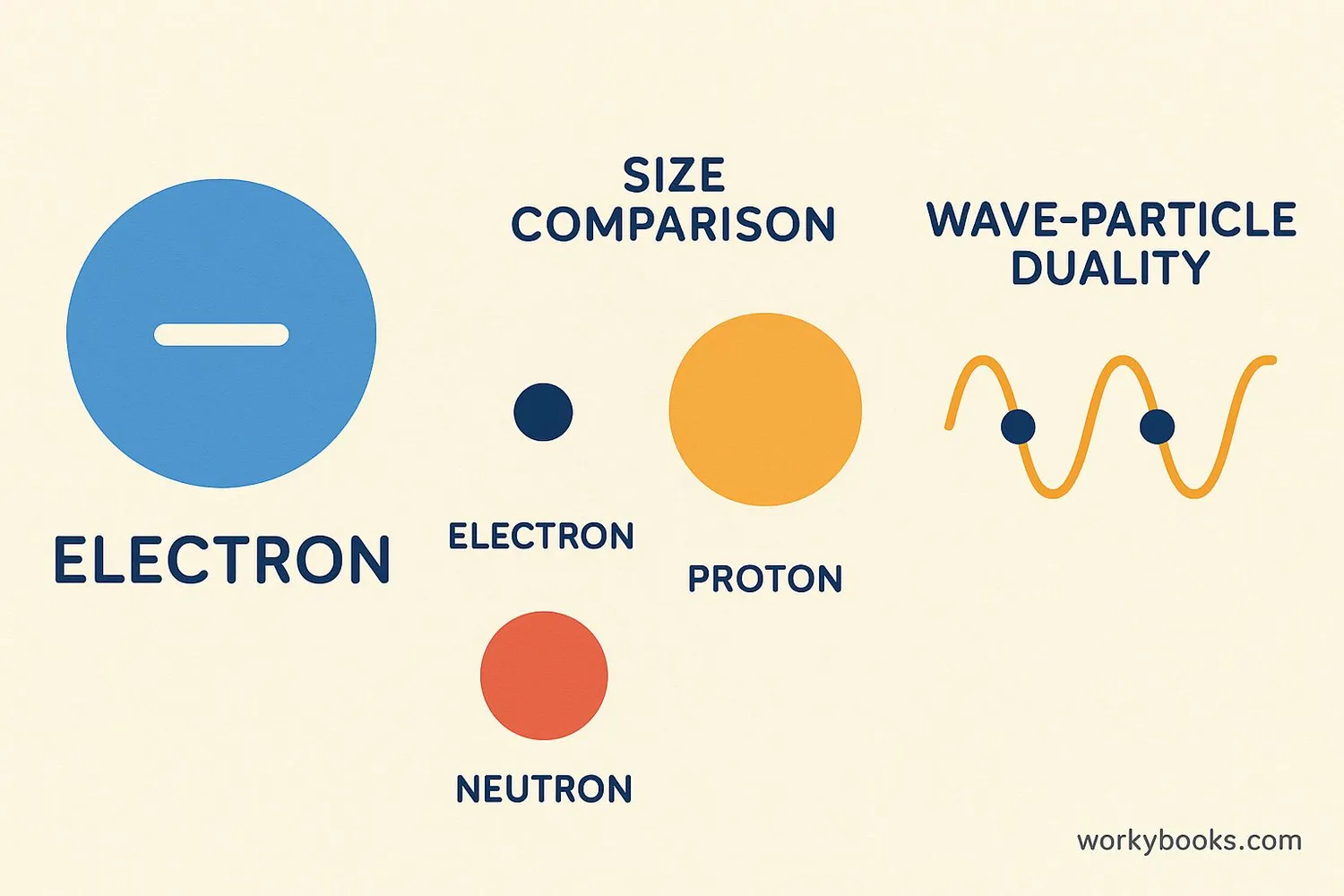
Electrons have some fascinating properties that make them unique:
Negative Charge
Electrons carry a negative electrical charge
Tiny Mass
Electrons are 1/1836 the mass of a proton
Dual Nature
Electrons behave as both particles and waves
According to quantum mechanics, we can't know exactly where an electron is at any moment - we can only describe the probability of finding it in different locations. This is why we show electrons as clouds around the nucleus rather than specific orbits.
Electron Flow and Electricity
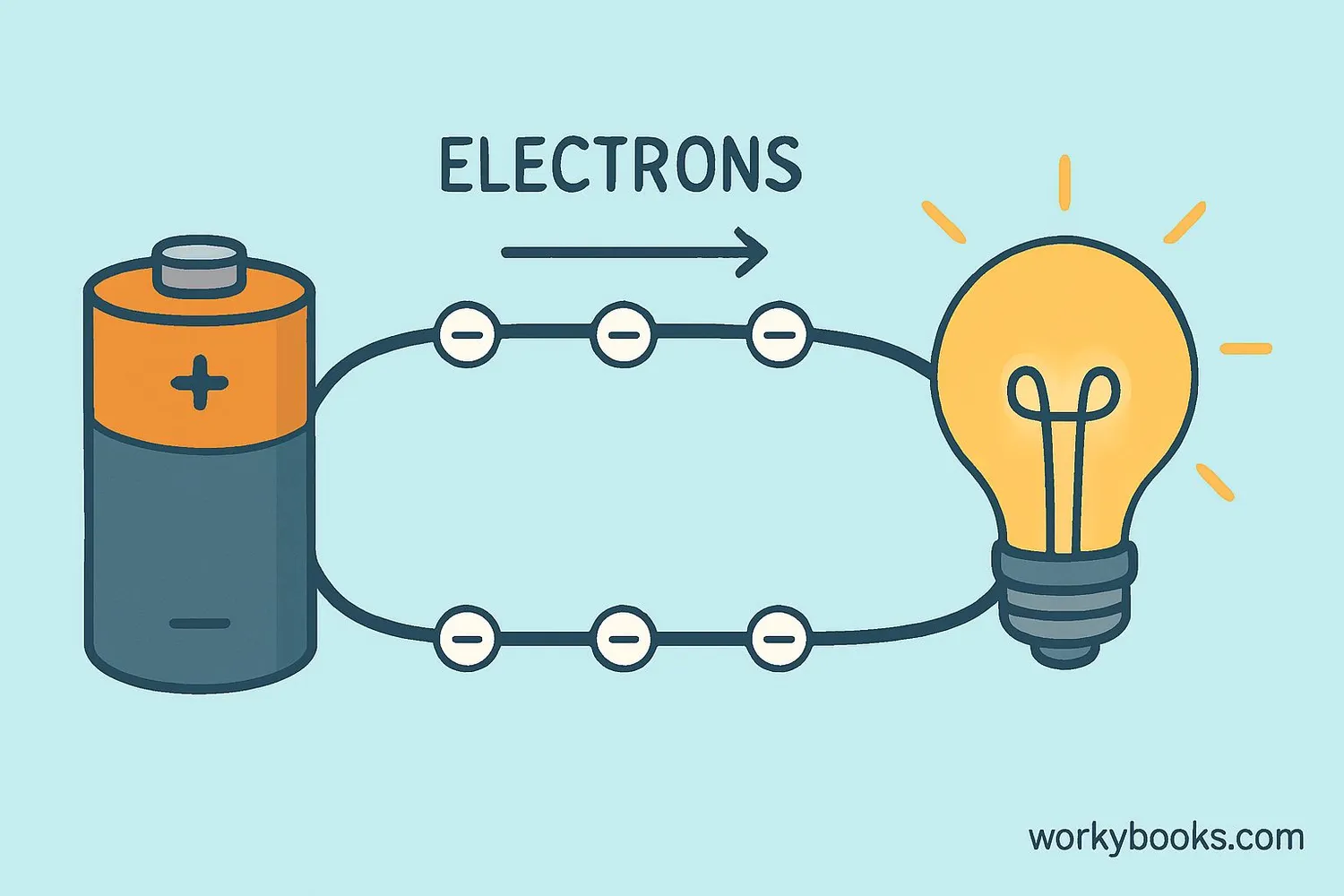
Electricity is essentially the controlled flow of electrons through a conductor like copper wire. When electrons move from one atom to another, they create an electric current that powers our devices.
How it works:
1. Electrons in the outer shells can move between atoms
2. When pushed by a voltage source (like a battery), electrons flow
3. This flow creates electrical current
4. The current can power lights, devices, and machines
Real-World Example
When you flip a light switch, you're completing a circuit that allows electrons to flow through the wires to the light bulb, causing it to glow!
Electron Quiz
Test your knowledge about electrons with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about electrons:
Fun Electron Trivia
Discover some amazing facts about electrons!
Speedy Particles
Electrons move incredibly fast - about 2,200 kilometers per second! That's nearly 1% the speed of light. At that speed, an electron could circle the Earth's equator in just 18 seconds!
Powering the World
The electricity powering your device right now is created by billions of electrons flowing through wires. A 60-watt light bulb uses about 18,700,000,000,000,000,000 electrons per second!
Chemical Bonds
Electrons are responsible for all chemical reactions! When atoms share or transfer electrons, they form chemical bonds. Without electrons, molecules like water couldn't exist.
Quantum Mystery
Electrons can be in two places at once! Quantum physics shows that electrons exist as probability waves until observed. This strange behavior is fundamental to modern electronics.





