Chemical Elements - Definition, Examples, Quiz, FAQ, Trivia
Discover the building blocks of everything around us!
What is a Chemical Element?
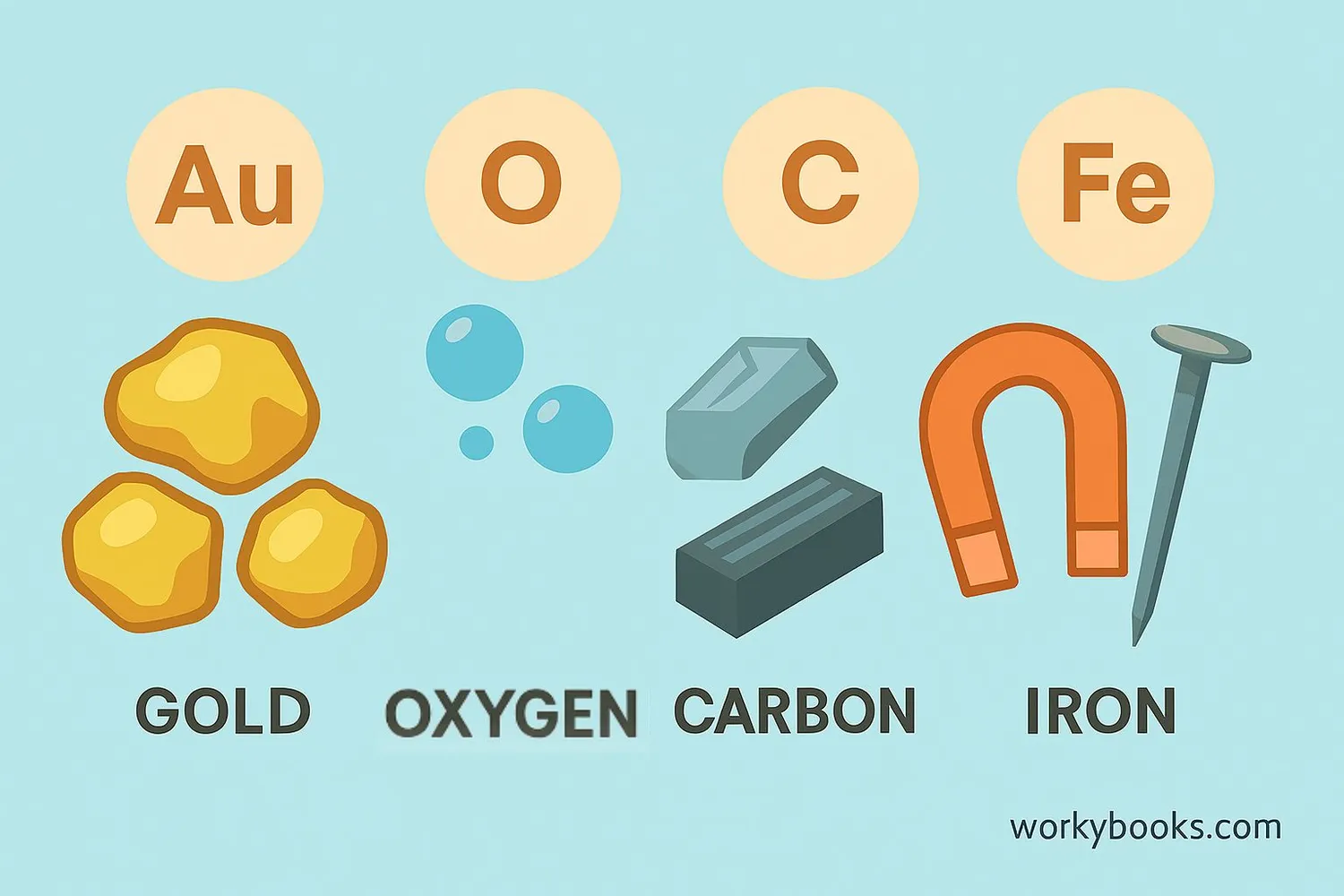
A chemical element is a pure substance made of only one type of atom. Elements are the basic building blocks of all matter in the universe. Everything around you - the air you breathe, the water you drink, and even your body - is made of different combinations of elements!
There are 118 known elements, but only about 90 occur naturally on Earth. Each element has its own unique properties and is represented by a one or two-letter symbol. For example, oxygen is O, gold is Au, and carbon is C. Elements cannot be broken down into simpler substances by ordinary chemical reactions.
Element Fact!
The human body is made mostly of just six elements: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus!
Atoms: Building Blocks of Elements
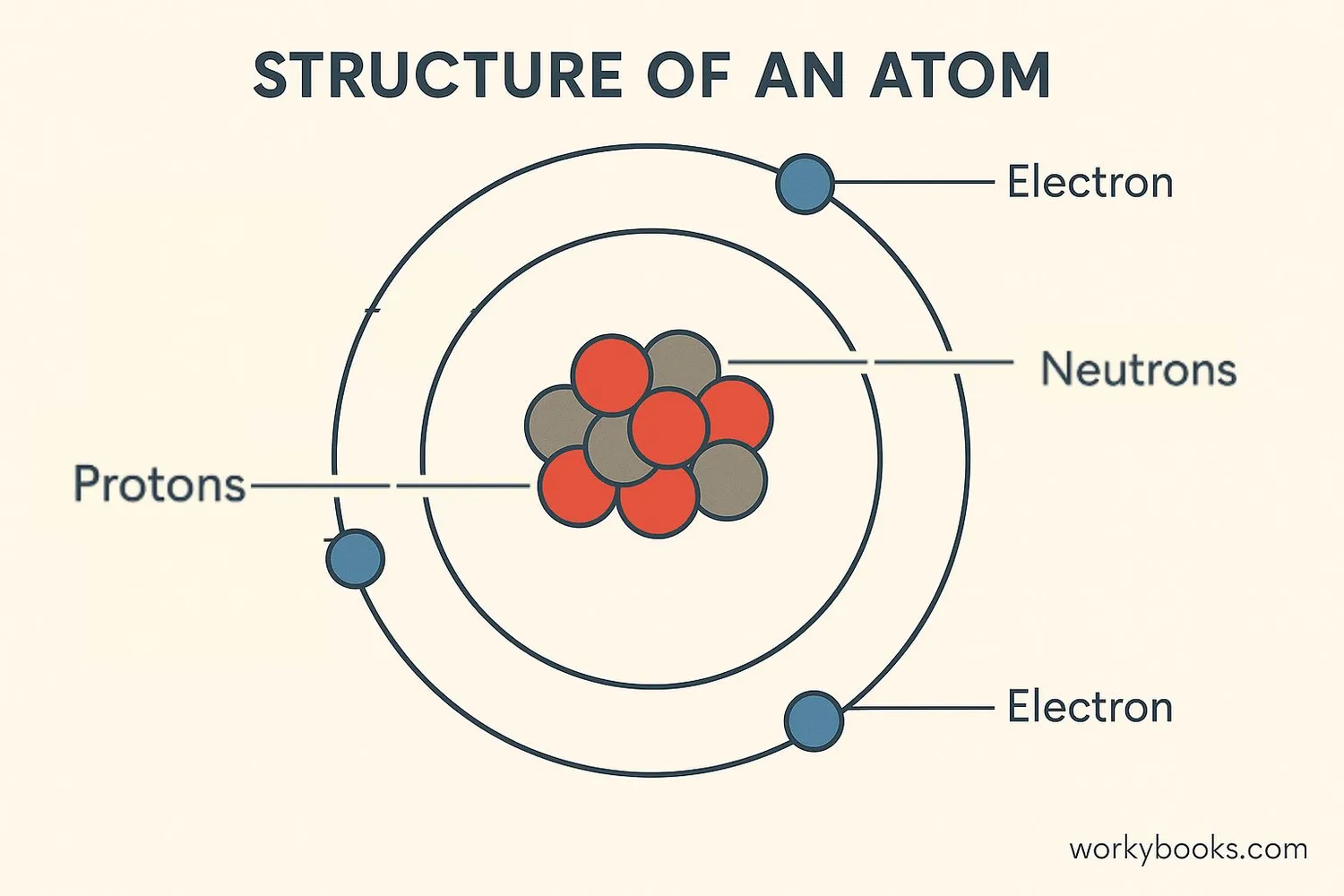
All elements are made of tiny particles called atoms. Atoms are so small that millions could fit on the head of a pin! Each atom consists of three types of even smaller particles:
Protons
Positively charged particles in the atom's nucleus
Neutrons
Neutral particles in the nucleus (no charge)
Electrons
Negatively charged particles orbiting the nucleus
The atomic number of an element tells you how many protons are in each atom. This number is unique to each element. For example, all hydrogen atoms have 1 proton (atomic number 1), while all oxygen atoms have 8 protons (atomic number 8).
The atomic mass is approximately the total number of protons and neutrons in an atom. Electrons are so small that they don't add much to the atom's mass.
The Periodic Table of Elements
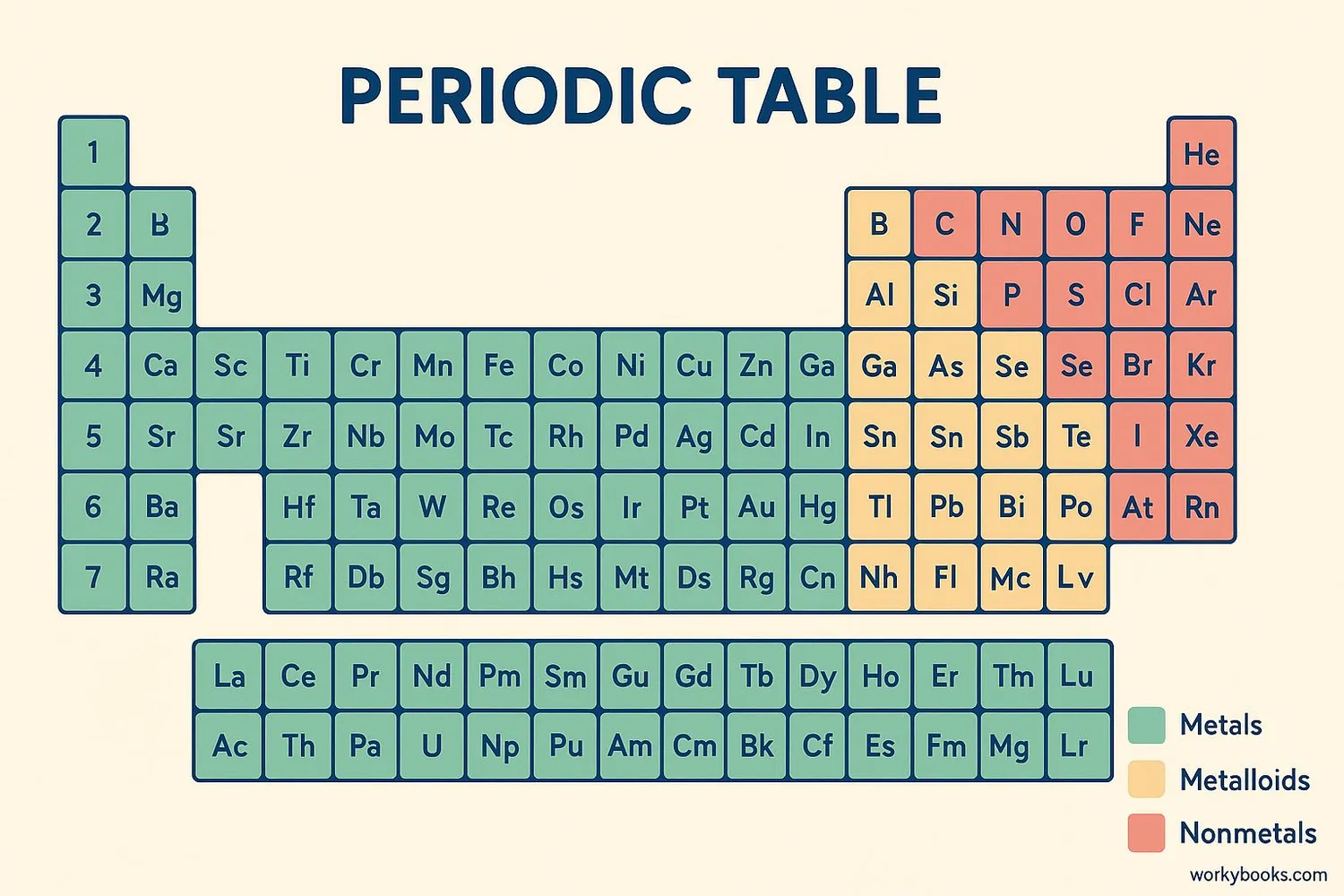
The periodic table is a special chart that organizes all the chemical elements in a specific way. It was created by Russian scientist Dmitri Mendeleev in 1869. Elements are arranged in order of increasing atomic number (number of protons) from left to right and top to bottom.
The table has rows called periods and columns called groups. Elements in the same group have similar chemical properties. For example, all elements in group 18 (the last column) are noble gases that don't easily react with other elements.
Periodic Table Fact!
Mendeleev left gaps in his original periodic table for elements that hadn't been discovered yet, correctly predicting their properties!
Element Properties: Metals, Nonmetals, and Metalloids
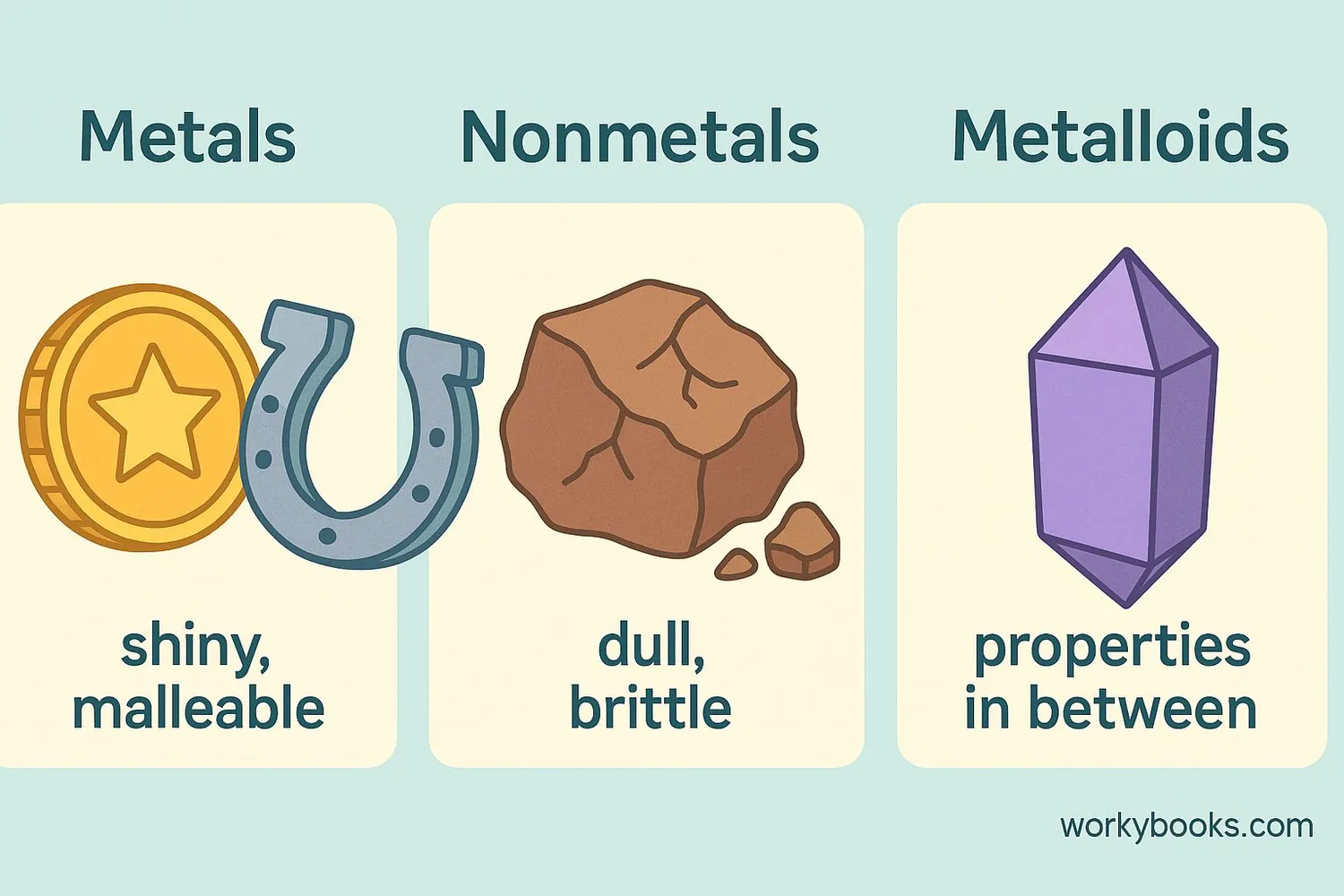
Elements can be classified into three main categories based on their physical and chemical properties:
Metals
Shiny, good conductors of heat and electricity, malleable (can be hammered into sheets)
Nonmetals
Dull appearance, poor conductors, brittle when solid
Metalloids
Have properties of both metals and nonmetals
Most elements are metals (about 75%). They're found on the left side and middle of the periodic table. Nonmetals are on the right side, and metalloids form a zigzag line between metals and nonmetals.
Elements can combine to form compounds. For example, sodium (a metal) and chlorine (a nonmetal) combine to form sodium chloride - table salt! Water is a compound of hydrogen and oxygen (H₂O).
Elements Quiz
Test your knowledge about chemical elements with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about chemical elements:
Fun Element Trivia
Discover some amazing facts about chemical elements!
Stardust Connection
Every atom in your body except hydrogen was created inside stars! When stars explode as supernovas, they spread these elements throughout the universe.
Rarest Natural Element
Astatine is the rarest naturally occurring element on Earth. Scientists estimate there's less than 1 gram of astatine in the Earth's crust at any time!
Liquid Elements
Only two elements are liquid at room temperature: mercury and bromine. Gallium melts in your hand (at 30°C), and cesium melts on a warm day (at 28°C).
Diamond Composition
Diamonds are made of just one element: carbon! The same element that makes up the graphite in your pencil, but arranged in a different crystal structure.





