Entropy - Definition, Examples, Quiz, FAQ, Trivia
Discover how disorder and randomness work in our universe
What is Entropy?
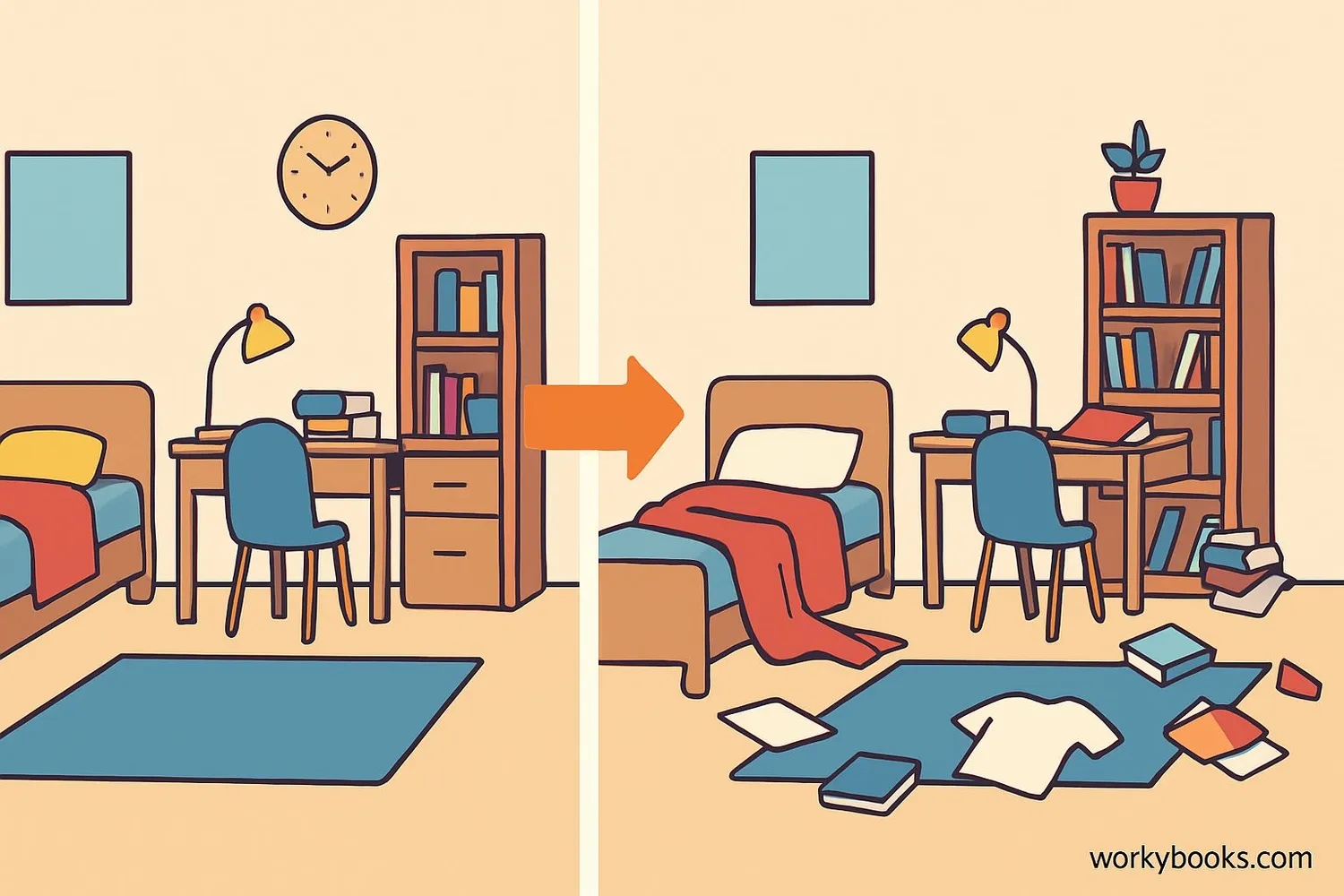
Entropy is a scientific way to measure disorder or randomness in a system. Imagine your bedroom - when it's clean, everything has its place. But over time, it naturally becomes messy. That's entropy in action! The term was invented by scientist Rudolf Clausius in the 1850s.
In science, entropy helps us understand why things happen in a certain way. It explains why ice melts in your drink, why your hot chocolate cools down, and why it's easier to make a mess than to clean it up. Entropy always increases in the universe, which means things naturally become more disordered over time.
Science Fact!
Entropy is sometimes called "time's arrow" because it shows the direction in which time moves - from order to disorder.
The Second Law of Thermodynamics

The Second Law of Thermodynamics is one of the most important rules in science, and it's all about entropy! This law states that in any energy transfer or transformation, the total entropy always increases. This has big consequences:
Heat engines (like car engines) can never be 100% efficient because some energy always becomes disordered heat that can't be used for work. This is why perpetual motion machines are impossible - they would violate the Second Law!
Energy Transfer
Energy moves from hot to cold objects
Entropy Increases
Disorder always increases in the universe
Irreversibility
Some processes only happen in one direction
Think about what happens when you drop an ice cube into a glass of water. The ice melts, and the water cools down a little. But have you ever seen the reverse happen? Ice cubes don't spontaneously form in a glass of water - that would require decreasing entropy, which violates the Second Law!
Energy Efficiency
The most efficient heat engines (like power plants) can only convert about 60% of heat energy into useful work because of entropy.
Entropy and Disorder
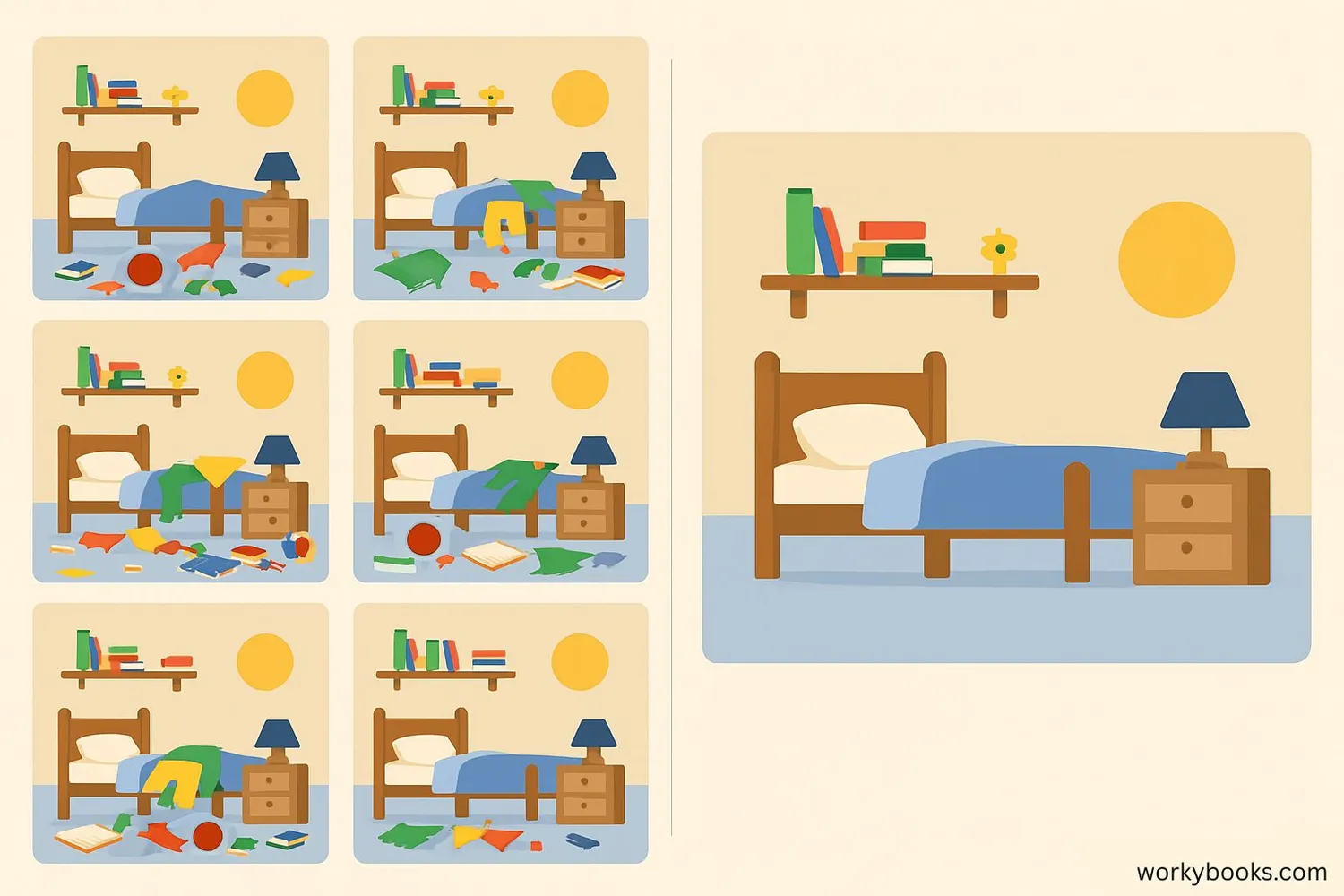
Scientist Ludwig Boltzmann explained entropy using probability. He showed that entropy measures the number of ways something can be arranged:
• Microstates: All the different ways particles can be arranged
• Macrostate: The overall state we observe (like messy or neat)
There are many more ways for your room to be messy than to be neat. Similarly, there are countless ways for air molecules to spread throughout a room, but only one way for them to all be in one corner. That's why entropy naturally increases - disorder is more probable!
Microstates
All possible arrangements of particles
Macrostate
The overall state we can observe
Probability
Disordered states are more likely
This statistical view of entropy helps us understand why:
• Ink spreads in water but never collects back together
• Sandcastles wash away but don't build themselves
• Air fills a room but doesn't gather in one corner
Each of these natural processes increases entropy because there are more disordered arrangements than ordered ones.
Entropy in Everyday Life

Entropy isn't just for scientists - we see it everywhere in daily life! Here are some common examples:
Heat Flow
Hot things cool down as heat spreads out
Mixing
Milk mixes with coffee but doesn't separate
Breaking
Cookies crumble but crumbs don't reassemble
Entropy also explains why we need to eat! When we use energy, we create disorder. Food gives us the energy we need to create order in our bodies and in our lives. Plants use sunlight to create order from disorder through photosynthesis, but they still increase the overall entropy of the universe.
Understanding entropy helps scientists develop better energy technologies and understand how the universe works. It's a fundamental concept in physics, chemistry, and even biology!
Entropy Quiz
Test your entropy knowledge with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about entropy:
Fun Entropy Trivia
Discover some amazing facts about entropy!
The Heat Death of the Universe
Scientists believe that entropy will eventually lead to the "heat death" of the universe, when all energy is evenly spread out and no more work can be done.
Information Theory
Computer scientists use entropy to measure information! The more random or surprising a message is, the higher its information entropy.
Biological Order
Your body constantly fights entropy by using energy to maintain order. When we stop doing this (when we die), entropy takes over and our bodies decompose.
Perpetual Motion
Entropy explains why perpetual motion machines are impossible. They would need to create energy from nothing or reverse entropy, both forbidden by physics laws.





