Intermolecular Forces - Definition, Examples, Quiz, FAQ, Trivia
Discover how molecules attract each other and affect the world around us
What are Intermolecular Forces?
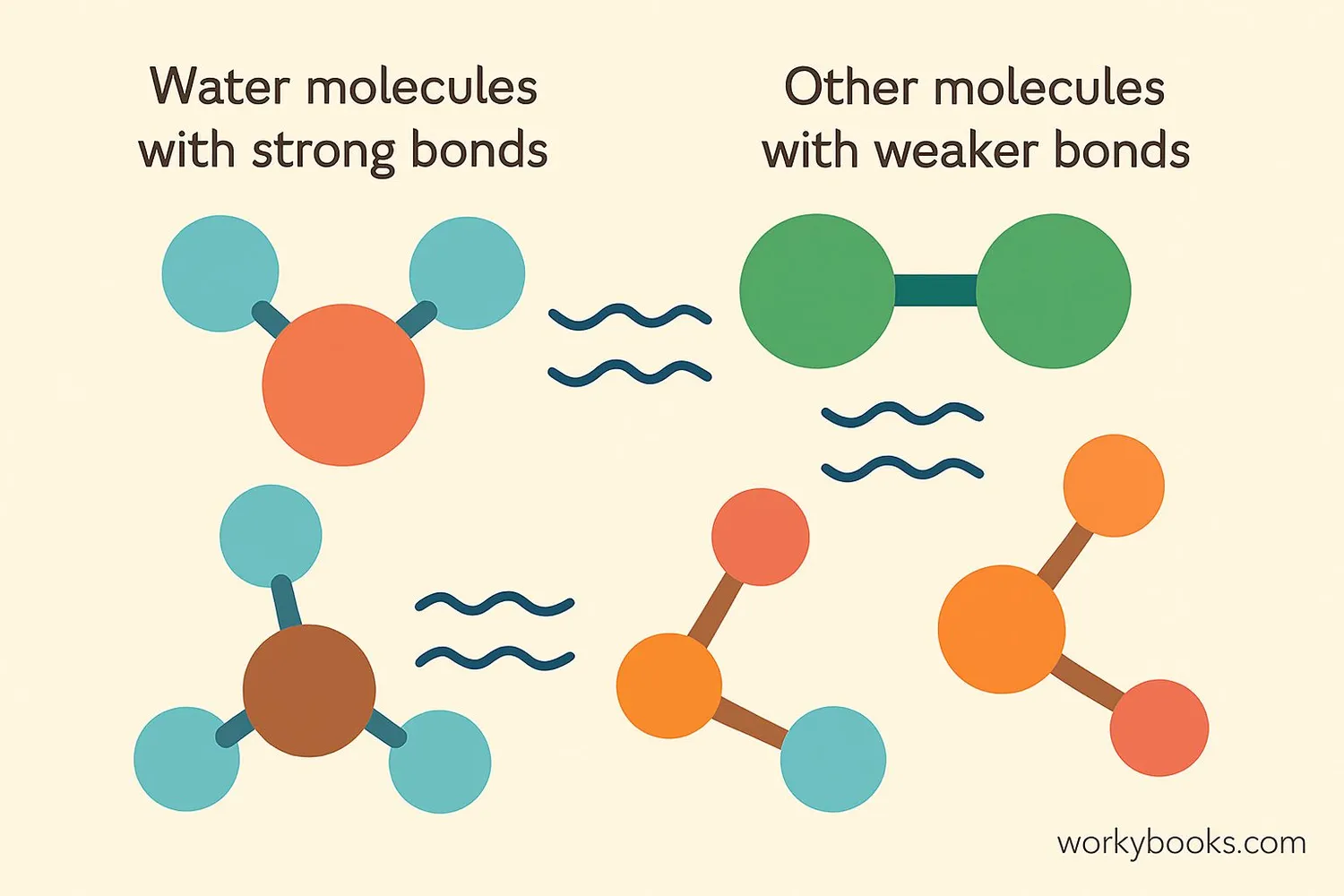
Intermolecular forces are the attractions between molecules that help them stick together. These forces are much weaker than the chemical bonds that hold atoms together within a molecule, but they're powerful enough to affect how substances behave!
Think of molecules like people at a party. The chemical bonds are like the strong connections between close friends or family members. Intermolecular forces are like the weaker attractions between people who just met - they might not be strong bonds, but they still affect how people group together!
Example
Water molecules stick together through hydrogen bonding, which is why water forms droplets and has surface tension that allows insects to walk on water!
Science Fact!
Intermolecular forces determine whether a substance is a solid, liquid, or gas at room temperature. Stronger forces mean higher melting and boiling points!
Types of Intermolecular Forces
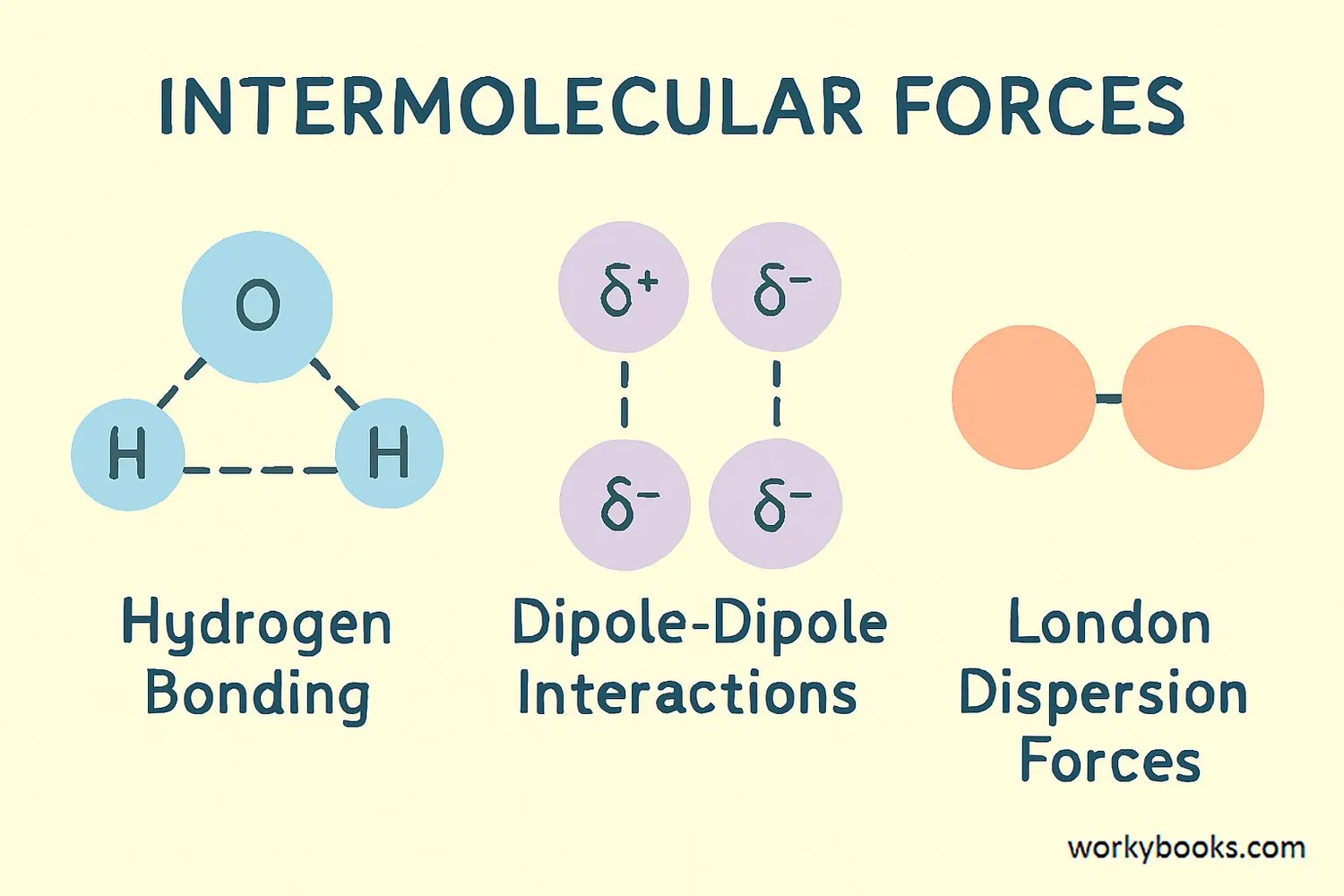
There are three main types of intermolecular forces, each with different strengths and characteristics:
London Dispersion Forces
The weakest force that occurs between all molecules when temporary electron imbalances create temporary dipoles
Dipole-Dipole Forces
Occur between polar molecules that have permanent positive and negative ends that attract each other
Hydrogen Bonding
A special strong type of dipole-dipole force that occurs when hydrogen is bonded to highly electronegative atoms
The strength of these forces increases from London dispersion (weakest) to dipole-dipole to hydrogen bonding (strongest). This is why substances with hydrogen bonding, like water, have much higher boiling points than similar-sized molecules without hydrogen bonding.
Example
Water (H₂O) has hydrogen bonding, which makes it liquid at room temperature, while methane (CH₄) only has London dispersion forces and is a gas at room temperature.
Real-World Importance
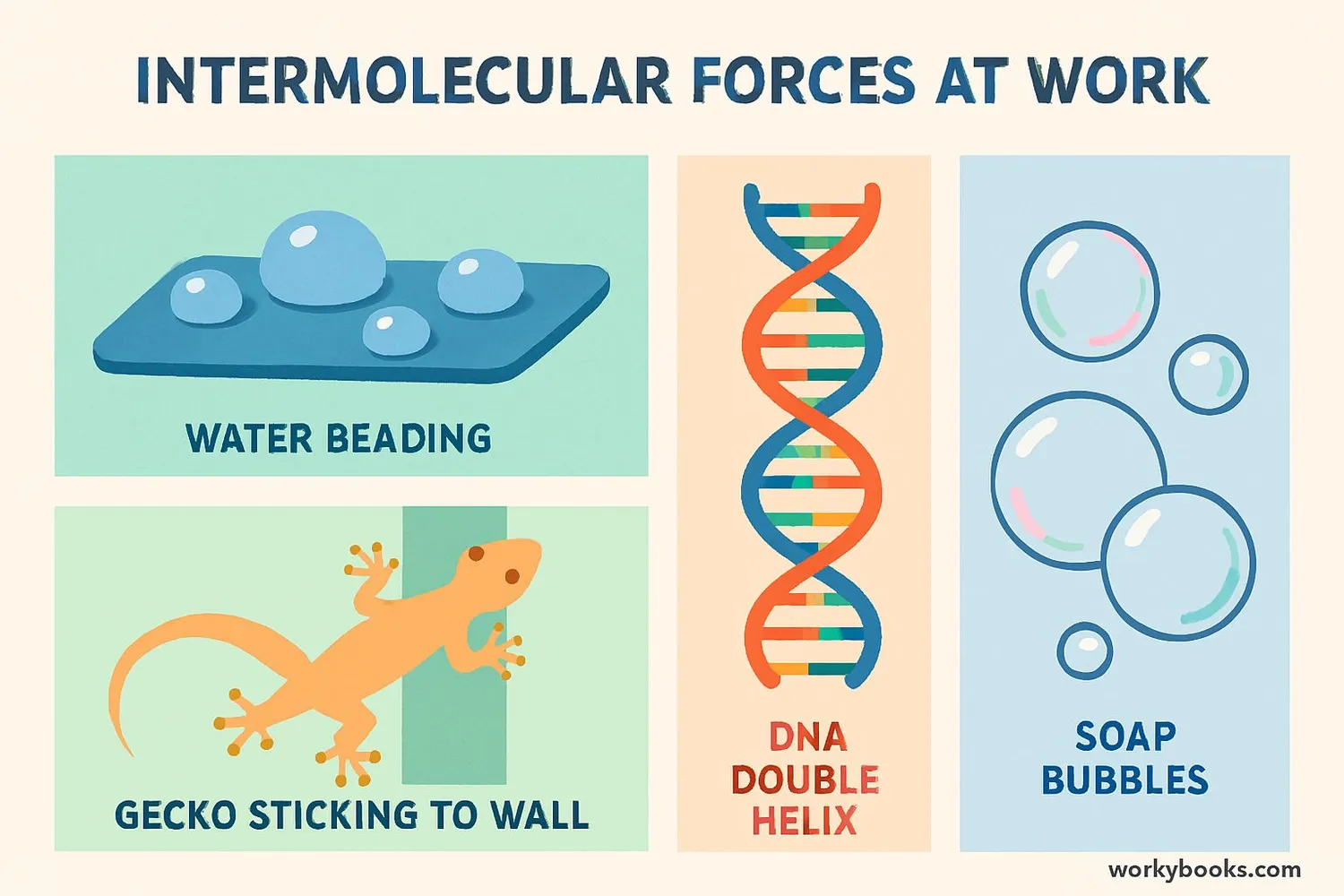
Intermolecular forces might be invisible, but they affect our world in countless important ways:
Surface Tension
Allows insects to walk on water and causes water to form droplets
DNA Structure
Hydrogen bonding holds the two strands of DNA together in its double helix
Adhesion
Allows glue to stick to surfaces and water to climb up thin tubes
These forces also explain:
• Why some substances dissolve in water while others don't
• How geckos can walk on ceilings (van der Waals forces)
• Why ice floats on water (hydrogen bonding creates open structure)
• How soap cleans grease (disrupts intermolecular forces)
Understanding intermolecular forces helps scientists design new materials, medicines, and technologies!
Gecko Feet Mystery Solved!
Geckos can climb smooth walls thanks to van der Waals forces between millions of tiny hairs on their feet and the surface!
Intermolecular Forces Knowledge Check
Test your understanding of intermolecular forces with this quiz. Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about intermolecular forces:
Science Facts About Intermolecular Forces
Discover some amazing facts about intermolecular forces!
DNA's Special Bonds
The double helix structure of DNA is held together by hydrogen bonds between base pairs. These relatively weak bonds allow DNA to "unzip" for replication and protein synthesis.
Water's Special Properties
Water has unusually high surface tension, boiling point, and specific heat capacity—all thanks to hydrogen bonding. This makes Earth perfectly suited for life as we know it.
Gecko Gravity-Defiers
A gecko can support its entire body weight with just one toe thanks to van der Waals forces! Their foot hairs split into hundreds of smaller hairs, maximizing surface contact.
Soap's Cleaning Power
Soap works because it disrupts intermolecular forces. One end of a soap molecule attracts water (hydrophilic) while the other end attracts oils and grease (hydrophobic), pulling them apart.





