Oxidizing Agents - Definition, Examples, Quiz, FAQ, Trivia
Discover how oxidizing agents work in chemical reactions!
What is an Oxidizing Agent?
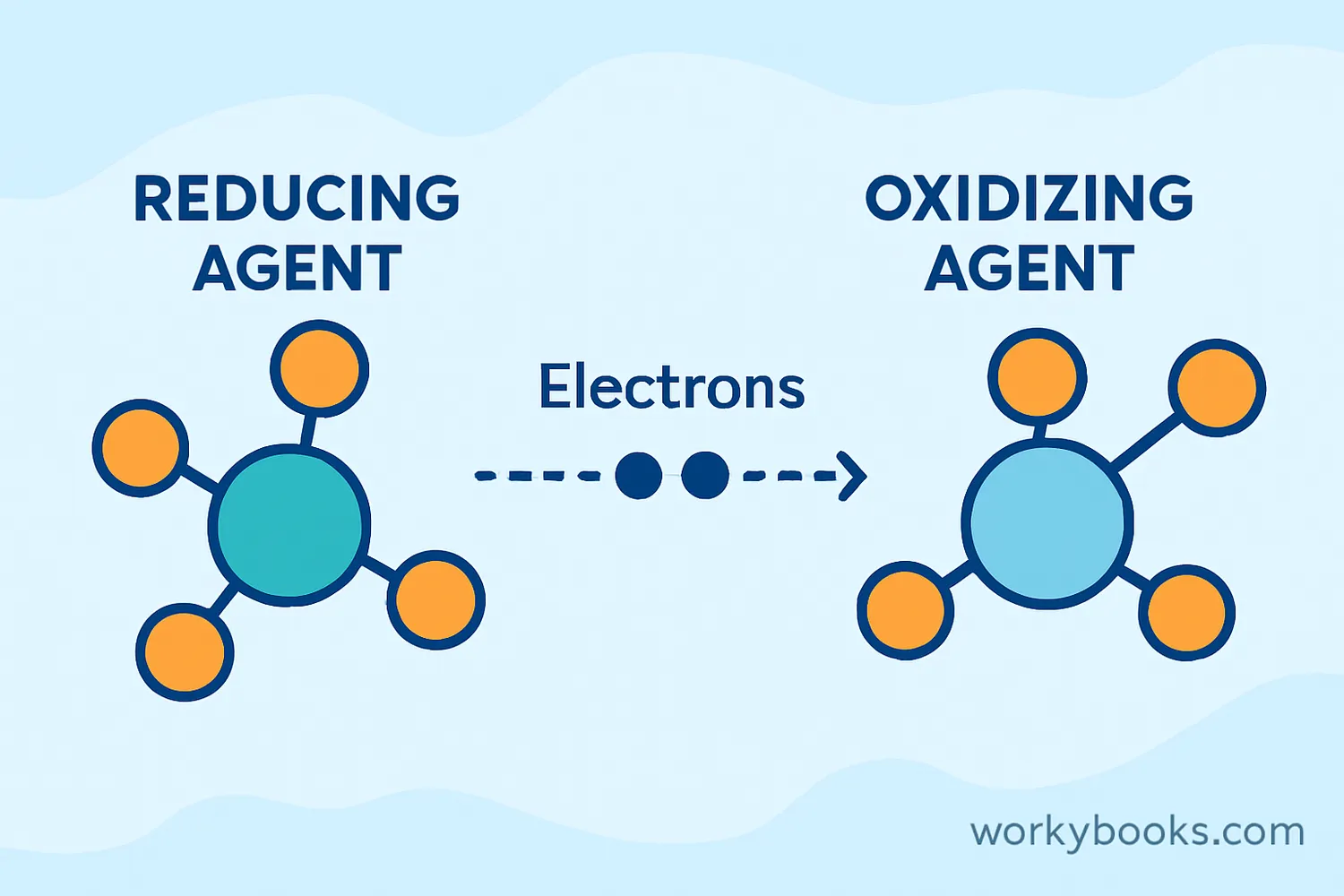
An oxidizing agent is a substance that helps other substances undergo oxidation by accepting electrons during a chemical reaction. Think of it as an "electron acceptor" that helps other chemicals change by taking their electrons.
In simpler terms, oxidizing agents are like the "helpers" in chemical reactions that allow other substances to combine with oxygen or lose electrons. This process is called oxidation, and it happens all around us - from rust forming on metal to the digestion of food in our bodies!
Common Oxidizing Agents
Oxygen is the most common oxidizing agent, but others include hydrogen peroxide, chlorine, and potassium permanganate.
How Oxidation Works
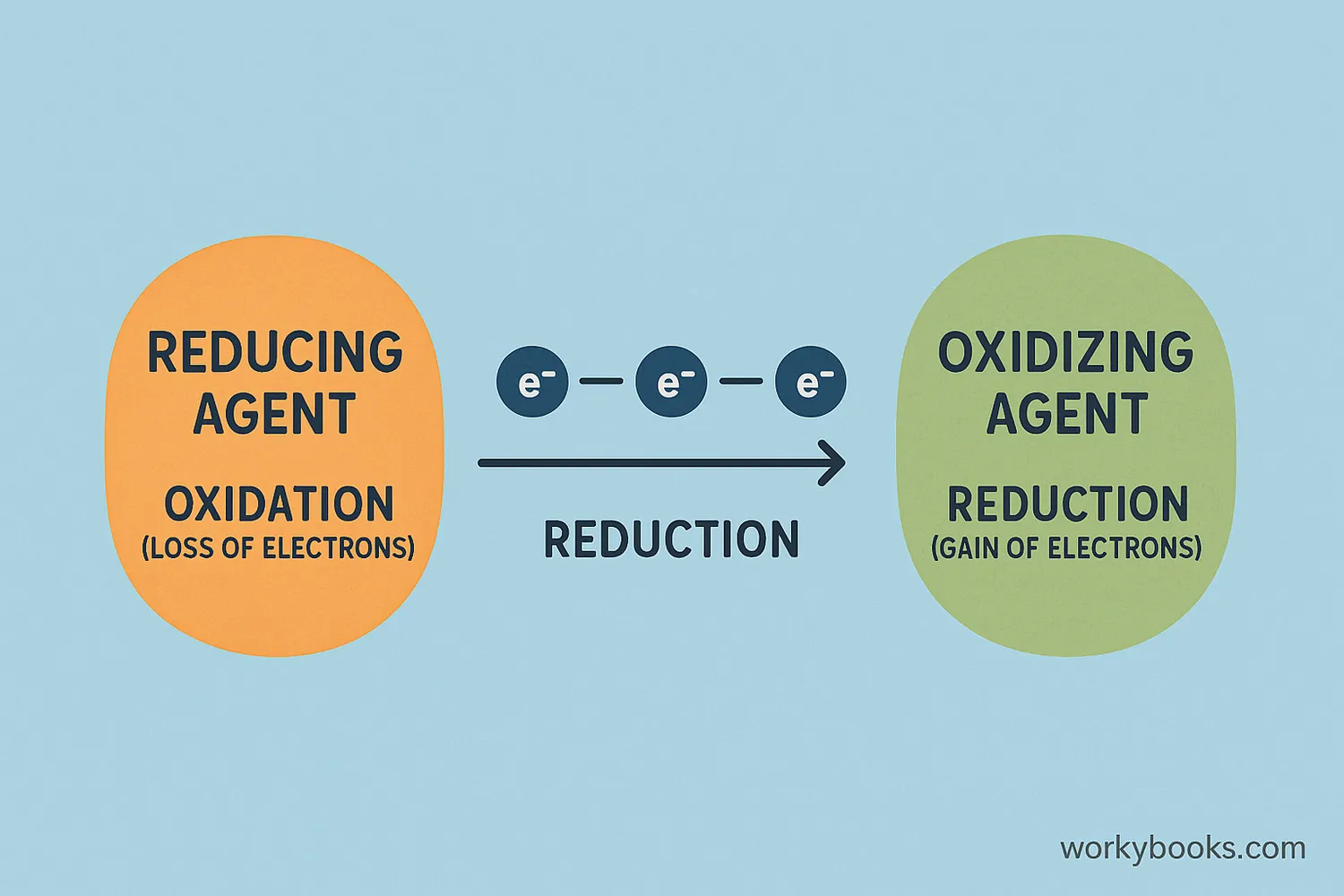
Oxidation and reduction always happen together in what chemists call "redox reactions." Here's how these reactions work:
Electron Transfer
One substance loses electrons (oxidation)
Electron Acceptance
Another substance gains those electrons (reduction)
Chemical Change
Both substances transform into new compounds
In every redox reaction:
• The substance that accepts electrons is the oxidizing agent
• The substance that donates electrons is the reducing agent
• The oxidizing agent gets reduced (gains electrons)
• The reducing agent gets oxidized (loses electrons)
Real-World Example
When iron rusts, iron atoms lose electrons (oxidation) to oxygen atoms, which gain those electrons (reduction). Oxygen is the oxidizing agent in this reaction.
Why Oxidizing Agents Are Important
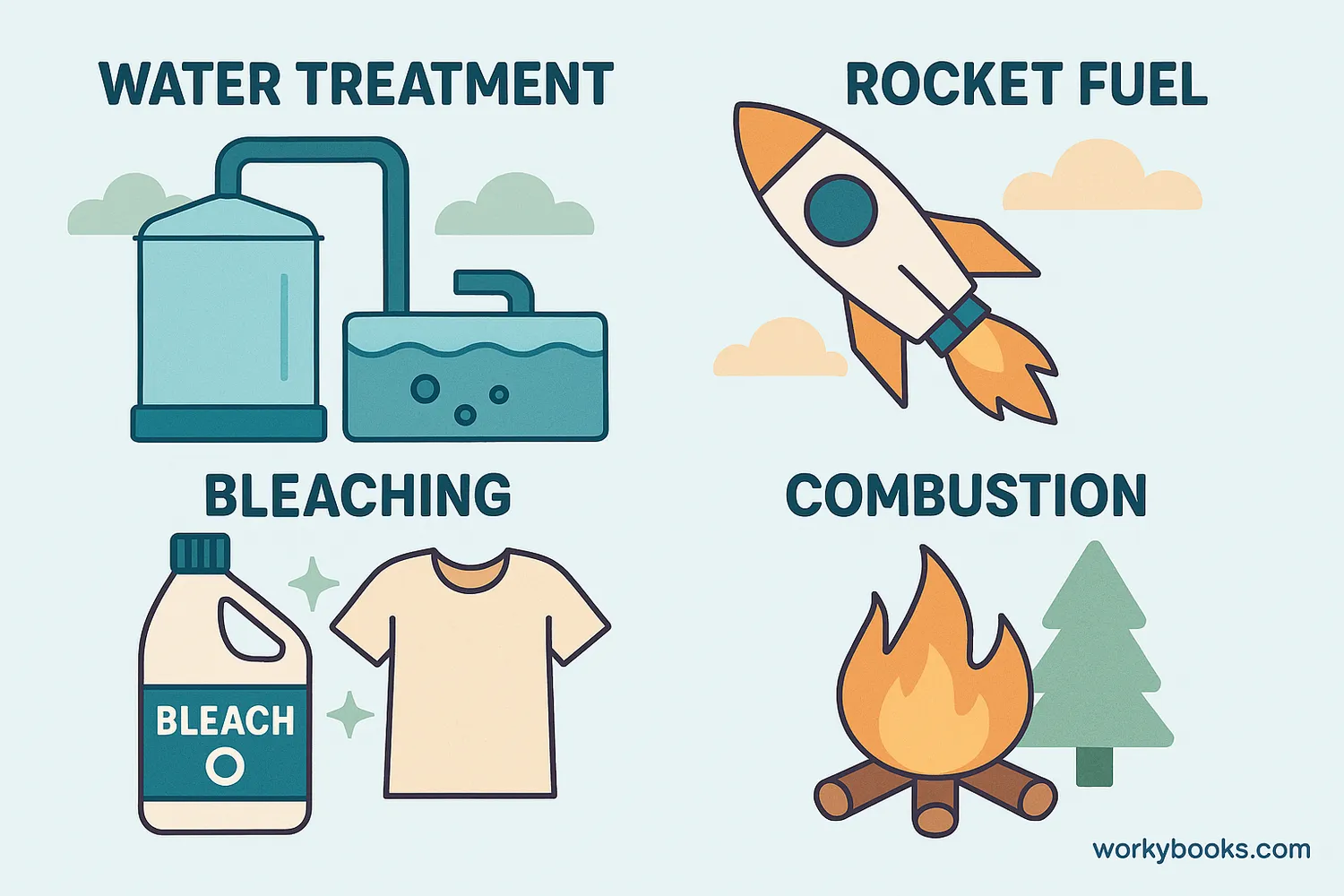
Oxidizing agents play crucial roles in many processes essential to our daily lives:
Combustion
Oxygen allows fuels to burn, providing heat and energy
Water Treatment
Chlorine oxidizes and kills harmful microorganisms
Rocket Fuel
Oxidizers help fuels burn in space where there's no oxygen
Other important uses include:
• Bleaching: Removing color from fabrics and paper
• Metallurgy: Extracting metals from their ores
• Batteries: Creating electrical energy through redox reactions
• Biological Processes: Cellular respiration uses oxygen to extract energy from food
Oxidizing Agents Quiz
Test your knowledge about oxidizing agents with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about oxidizing agents:
Science Facts About Oxidizing Agents
Discover some interesting facts about oxidizing agents!
Rocket Power
Rockets need oxidizing agents to burn fuel in space where there's no oxygen. Ammonium perchlorate is often used as the oxidizer in solid rocket boosters!
Ancient Bleaching
Ancient Egyptians used sunlight (which contains ultraviolet radiation, a natural oxidizing agent) to bleach linen fabrics over 4,000 years ago!
Plant Defenses
Some plants produce hydrogen peroxide (an oxidizing agent) when injured to help protect against invading bacteria and fungi!
Strongest Oxidizer
Fluorine is the strongest known oxidizing agent—it's so reactive that it can cause glass, metals, and even water to burn!





