Solubility - Definition, Examples, Quiz, FAQ, Trivia
Discover how substances dissolve and form solutions!
What is Solubility?
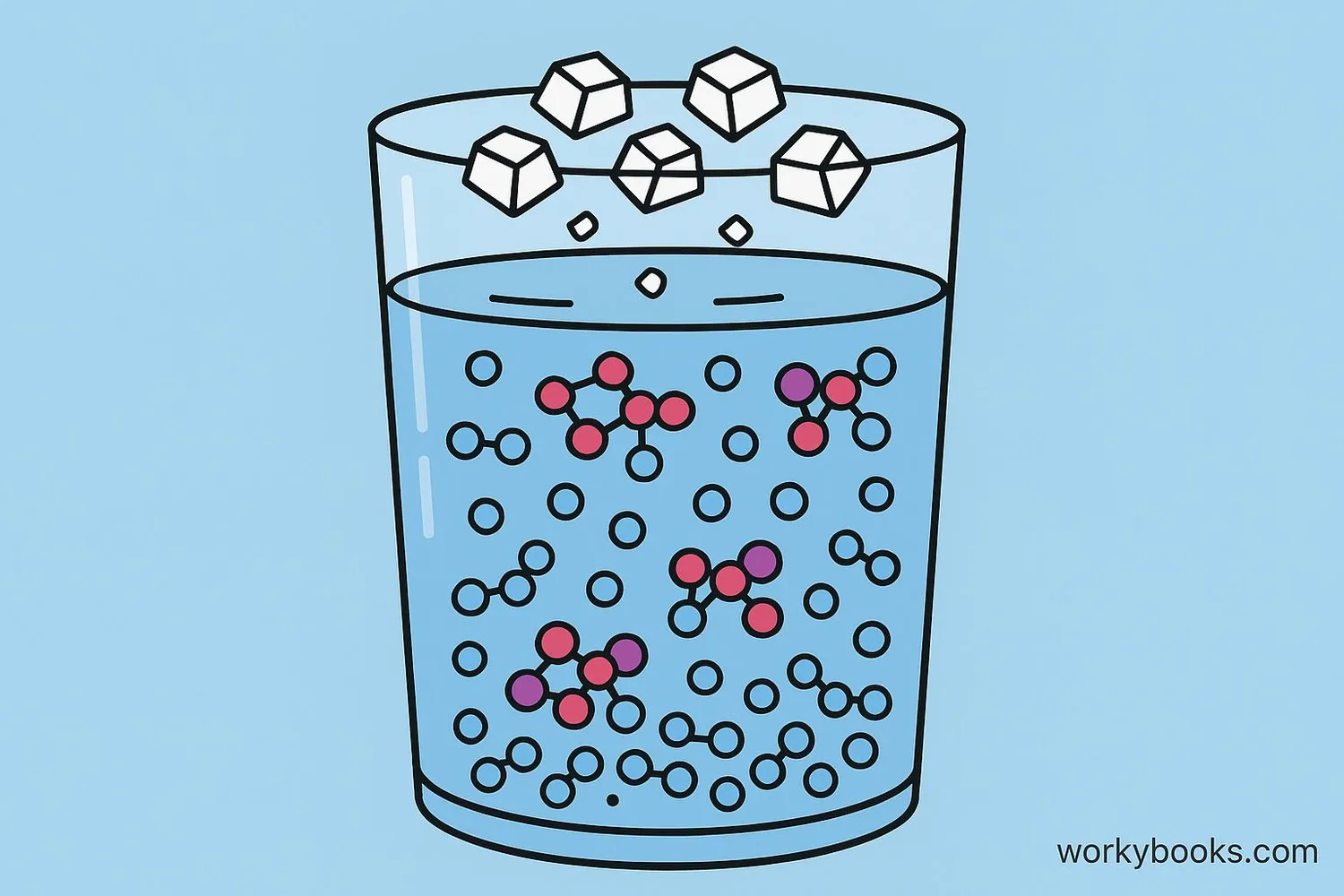
Solubility is a measure of how well a substance (called the solute) can dissolve in another substance (called the solvent). When a substance dissolves, it breaks down into tiny particles that mix evenly throughout the solvent, creating a solution.
Think about stirring sugar into your tea. The sugar disappears, but you can still taste its sweetness because it has dissolved and spread evenly throughout the liquid. That's solubility in action!
Key Terms
Solute: The substance that dissolves (e.g., sugar, salt)
Solvent: The substance that does the dissolving (e.g., water)
Solution: The mixture of solute and solvent
Different substances have different solubility levels. Salt dissolves easily in water (highly soluble), while sand does not (insoluble). The principle "like dissolves like" explains why oil and water don't mix - oil molecules are nonpolar while water molecules are polar.
Types of Solutions
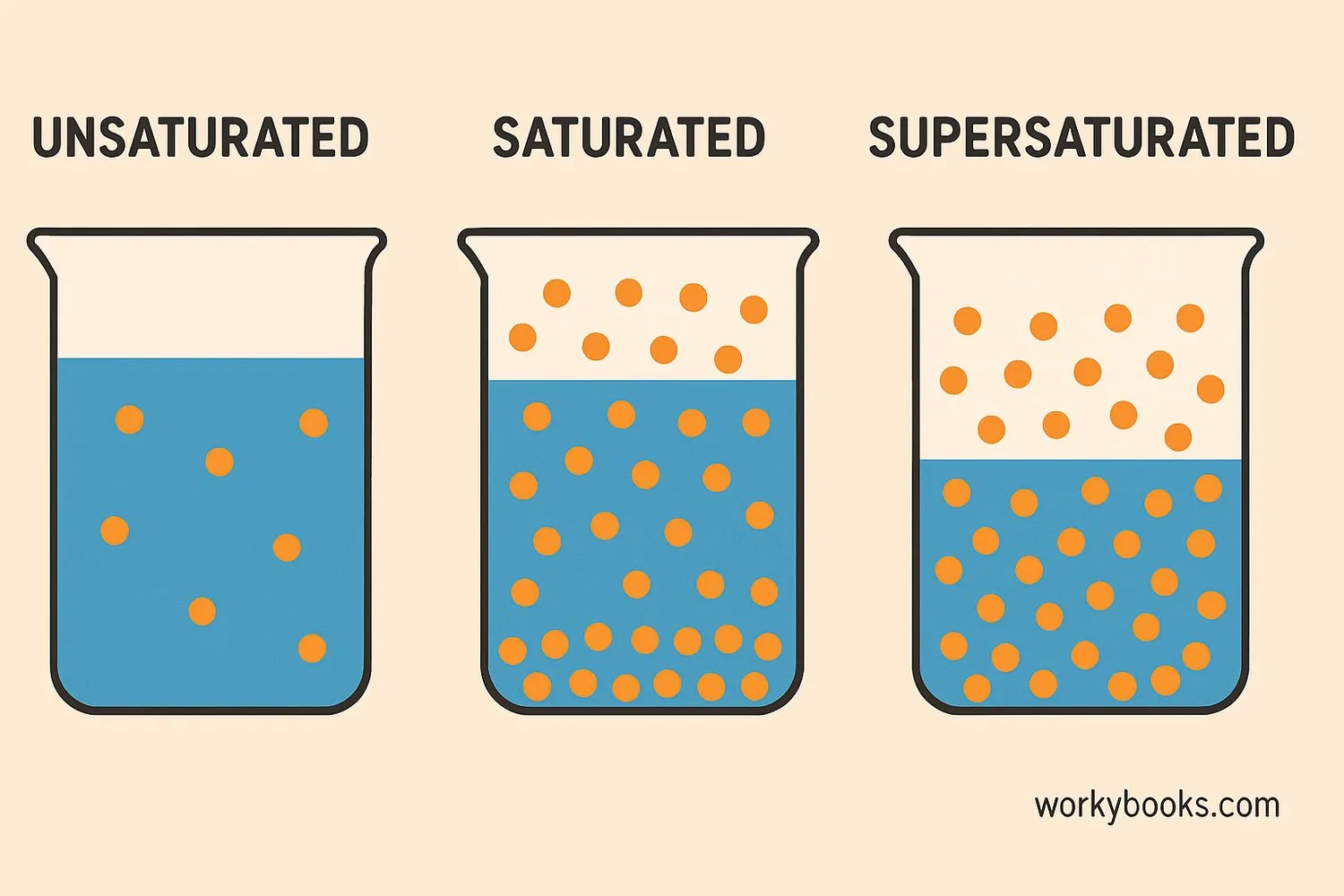
Solutions can be classified based on how much solute they contain:
Unsaturated Solution
A solution that can dissolve more solute at a given temperature
Saturated Solution
A solution that contains the maximum amount of solute that can dissolve at a given temperature
Supersaturated Solution
A solution that contains more dissolved solute than it normally could at that temperature
A saturated solution is in dynamic equilibrium - solute particles are dissolving at the same rate as they are crystallizing. If you add more solute to a saturated solution, it won't dissolve but will settle at the bottom.
The Dissolution Process
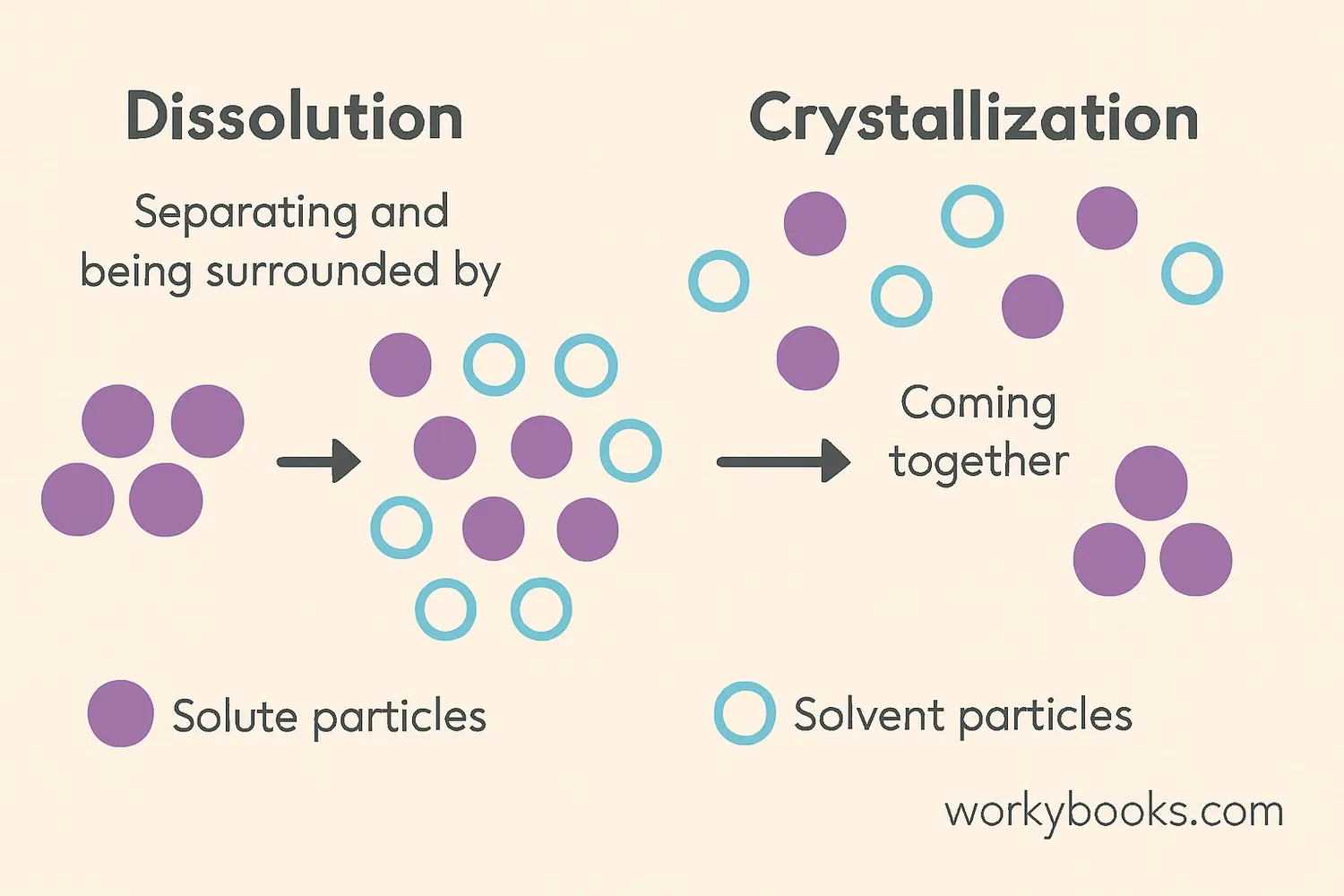
Dissolution is the process where solute particles separate and become surrounded by solvent particles. This happens through several steps:
The Dissolution Process
1. Solvent molecules surround the solute particles
2. Solvent molecules pull solute particles apart
3. Solute particles disperse evenly throughout the solvent
The opposite process is crystallization, where dissolved solute particles come back together to form solid crystals. In a saturated solution, dissolution and crystallization happen at the same rate, creating a solubility equilibrium.
This balance explains why sugar stops dissolving in your tea when the solution becomes saturated - the sugar crystals form as quickly as they dissolve!
Factors Affecting Solubility
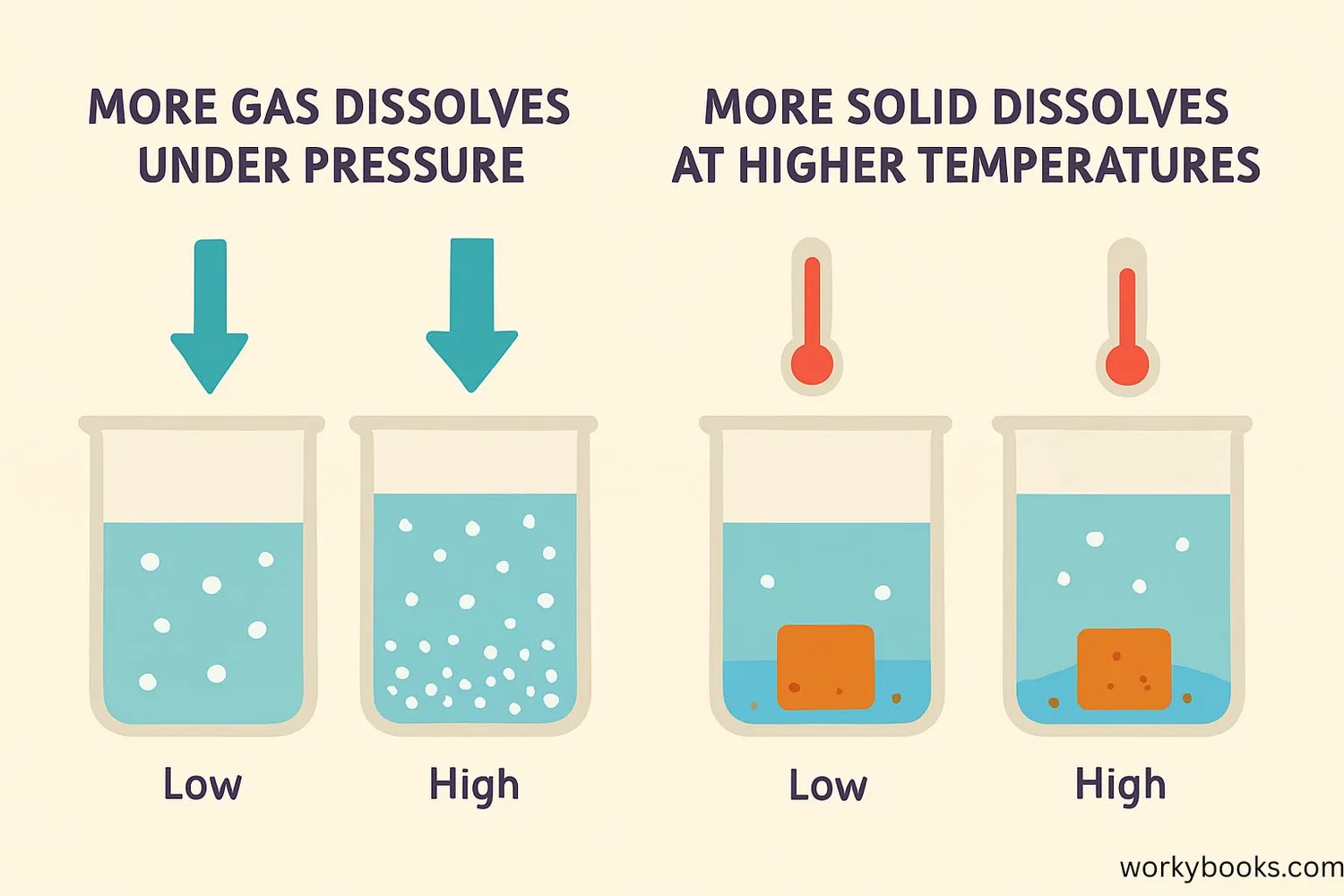
Several factors influence how much solute can dissolve in a solvent:
Temperature
For most solids, solubility increases with temperature. For gases, solubility decreases as temperature increases.
Pressure
Pressure mainly affects gas solubility. Higher pressure forces more gas molecules to dissolve (Henry's Law).
Nature of Solute and Solvent
Polar solvents dissolve polar solutes, nonpolar solvents dissolve nonpolar solutes ("like dissolves like").
Le Chatelier's Principle explains why solubility changes with temperature and pressure. When we increase temperature, the system counteracts by absorbing heat, which for dissolution of solids is an endothermic process, so more solid dissolves. For gases, dissolution is exothermic, so less gas dissolves at higher temperatures.
Solubility Quiz
Test your knowledge with this solubility quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about solubility:
Solubility Trivia
Discover amazing facts about solubility!
Ocean Salinity
The world's oceans contain enough salt to cover all continents with a layer over 500 feet thick! This salt dissolves in water through the water cycle.
Hot Springs Minerals
Hot springs have colorful mineral deposits because hot water can dissolve more minerals. As the water cools, minerals crystallize, creating beautiful formations.
Medical Solutions
Doctors use solubility principles to create medicines. Some drugs are designed to dissolve quickly, while others dissolve slowly for extended release in the body.
Cave Formation
Limestone caves form through solubility! Rainwater dissolves carbon dioxide to form weak carbonic acid, which slowly dissolves limestone over thousands of years.





