Transition Metals - Definition, Examples, Quiz, FAQ, Trivia
Discover the special metals that make our world colorful and strong!
What Are Transition Metals?
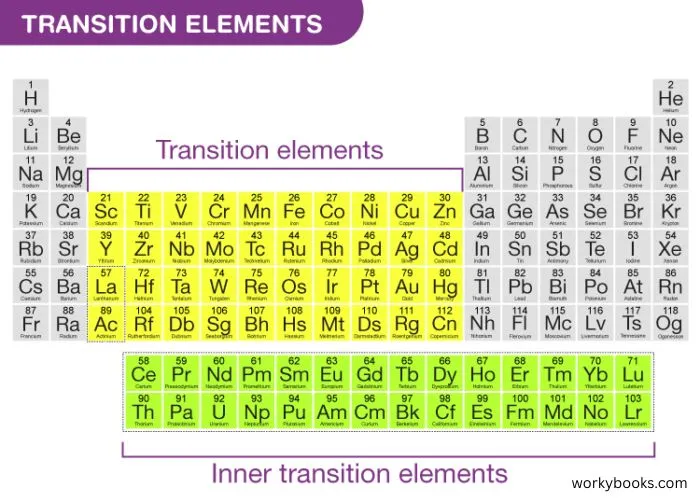
Transition metals are a group of elements found in the middle of the periodic table. They're called "transition" metals because they form a bridge between the metals on the left and the non-metals on the right.
These special metals include familiar elements like iron, copper, gold, and silver. They're known for being shiny, conducting electricity and heat well, and having high melting points. Many transition metals can form compounds with beautiful colors, which is why they're often used in paints and dyes.
Did You Know?
About 65% of all elements are metals, and most of these are transition metals!
Properties of Transition Metals

Transition metals have several special properties that make them useful for many applications:
Colorful Compounds
They form brightly colored compounds used in paints and ceramics
Good Conductors
They conduct electricity and heat very well
Malleable
They can be hammered into thin sheets without breaking
Ductile
They can be stretched into wires
Magnetic
Some (like iron, cobalt, and nickel) are magnetic
Another important property is that transition metals often have multiple oxidation states, which means they can form different types of chemical bonds. This allows them to create many different compounds with various colors and properties.
Variable Valency
Iron can form two different ions: Fe²⁺ (ferrous) and Fe³⁺ (ferric), which explains why rust can have different colors!
Examples of Transition Metals

There are many transition metals, but here are some of the most common and important ones:
Iron (Fe)
Used in steel production, magnets, and hemoglobin in blood
Copper (Cu)
Used in electrical wiring, plumbing, and coins
Gold (Au)
Used in jewelry, electronics, and as a monetary standard
Silver (Ag)
Used in jewelry, photography, and as an antimicrobial agent
Zinc (Zn)
Used to galvanize steel and in batteries
Chromium (Cr)
Used to make stainless steel and for chrome plating
Uses of Transition Metals
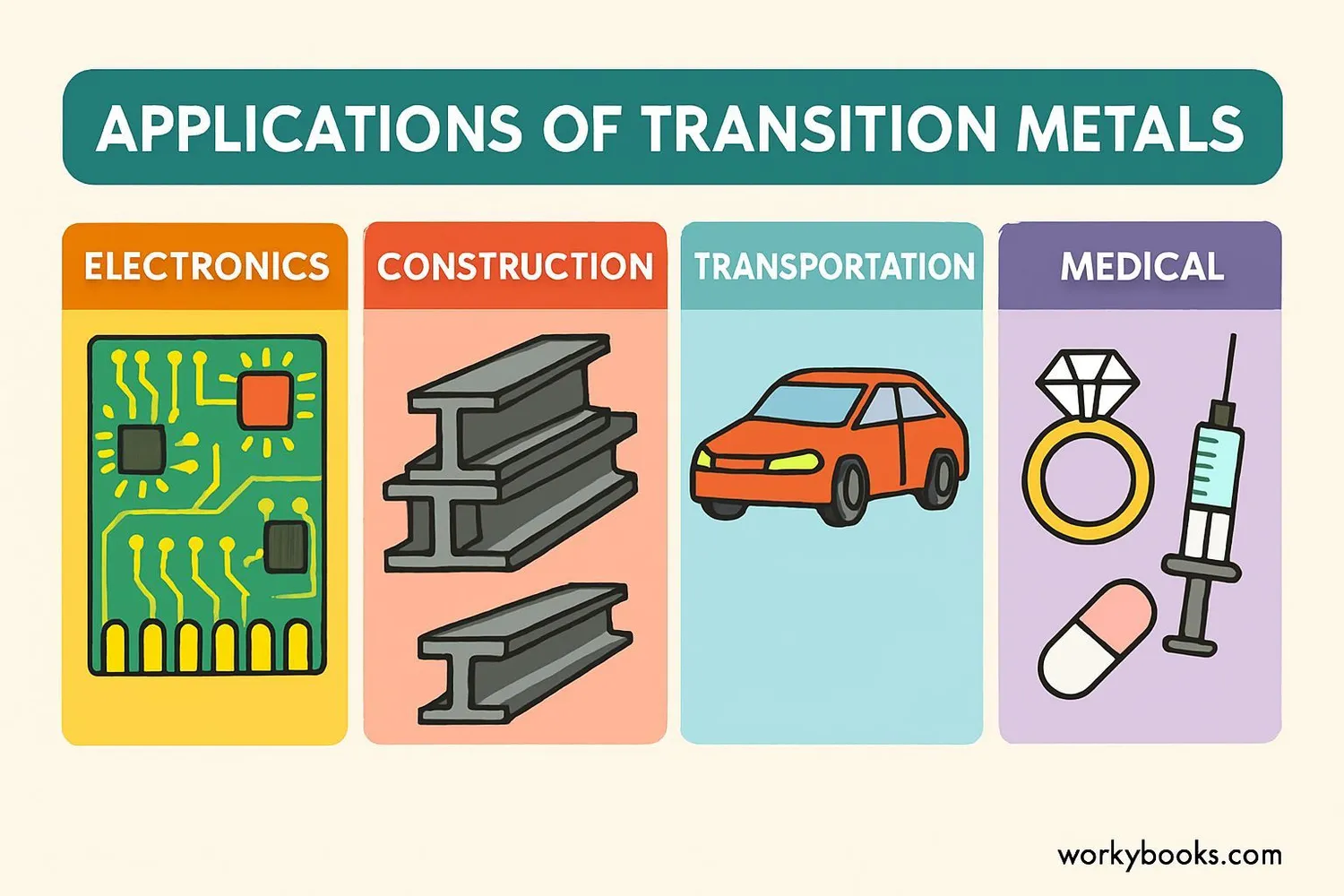
Transition metals are essential to modern life. Here are some of their important uses:
Construction
Iron and steel are used in buildings, bridges, and infrastructure
Electronics
Copper wiring, gold connectors, and silver contacts in devices
Transportation
Steel in vehicles, platinum in catalytic converters
Pigments
Compounds of chromium, cobalt, and cadmium create colorful paints
Medicine
Iron in hemoglobin, titanium in implants, platinum in cancer drugs
Currency
Copper, nickel, and zinc in coins; gold and silver for bullion
Without transition metals, we wouldn't have many of the technologies and conveniences we rely on every day. From the buildings we live in to the devices we use, transition metals play a crucial role in modern society.
Transition Metals Quiz
Test your knowledge about transition metals with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about transition metals:
Fun Transition Metals Trivia
Discover some amazing facts about transition metals!
Golden Marvel
All the gold ever mined in human history would fit into just three Olympic-sized swimming pools! Gold is so malleable that a single ounce can be stretched into a wire 50 miles long.
Iron Core
Earth's core is mostly made of iron, which creates our planet's magnetic field. This magnetic field protects us from harmful solar radiation and makes compass navigation possible.
Platinum Power
Platinum is used in catalytic converters in vehicles to reduce harmful emissions. About 50% of all platinum mined each year is used for this purpose, helping to keep our air cleaner.
Titanium Strength
Titanium has the highest strength-to-density ratio of any metal. It's as strong as steel but about 45% lighter, which is why it's used in aircraft, spacecraft, and high-performance sports equipment.





