Ozone Layer - Definition, Examples, Quiz, FAQ, Trivia
Discover Earth's protective shield against harmful ultraviolet radiation
What is the Ozone Layer?
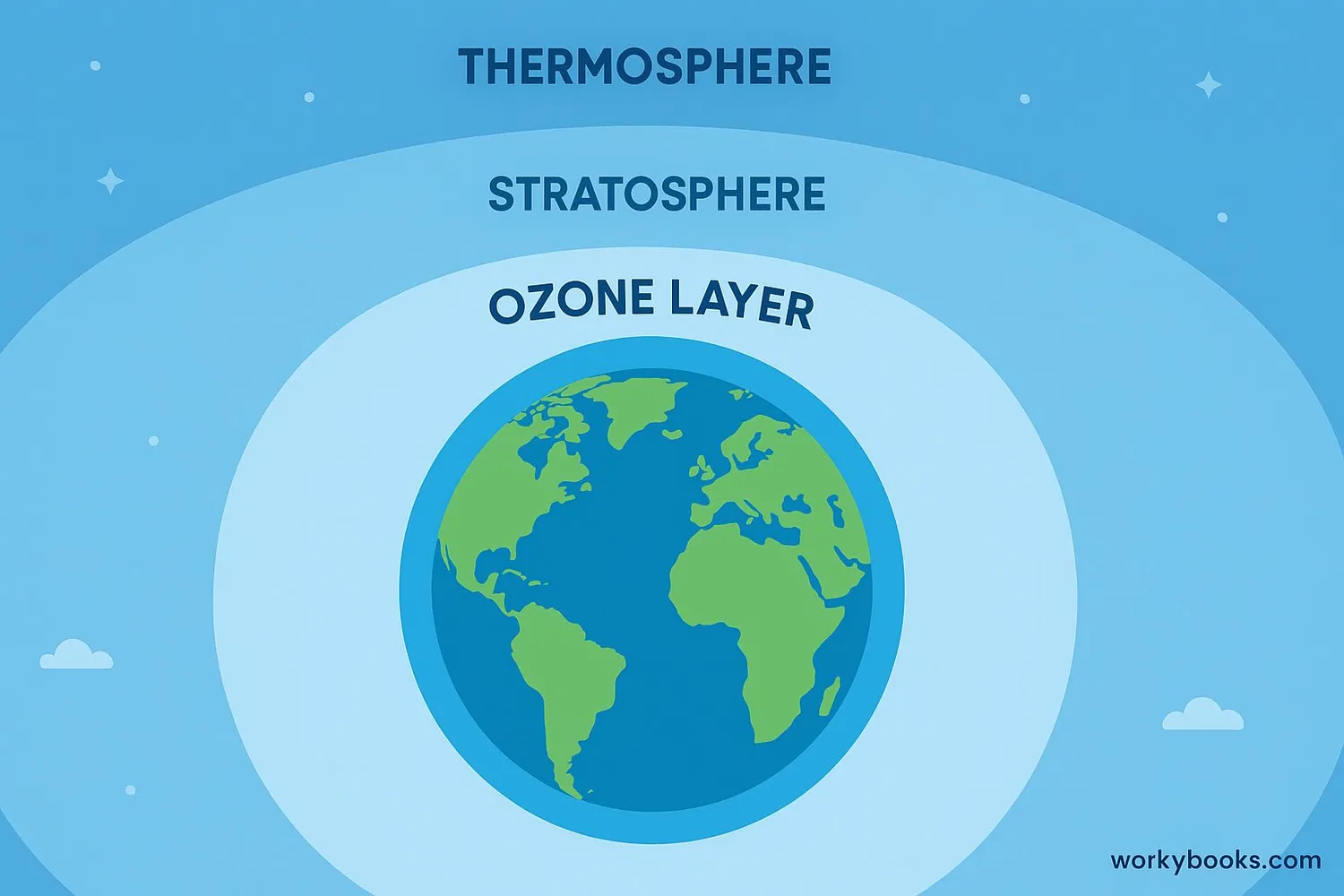
The ozone layer is a special part of Earth's atmosphere that acts like a giant shield around our planet. It's located in the stratosphere, about 10-30 miles above the Earth's surface. This layer contains a high concentration of ozone molecules (O₃) that absorb and block most of the sun's harmful ultraviolet radiation.
Think of the ozone layer as Earth's sunscreen! Just like sunscreen protects your skin from sunburn, the ozone layer protects all living things on Earth from dangerous UV rays. Without it, life as we know it wouldn't be possible.
Definition Fact!
Ozone is a special form of oxygen where three oxygen atoms bond together (O₃), unlike the oxygen we breathe which has two atoms (O₂).
How the Ozone Layer Protects Us
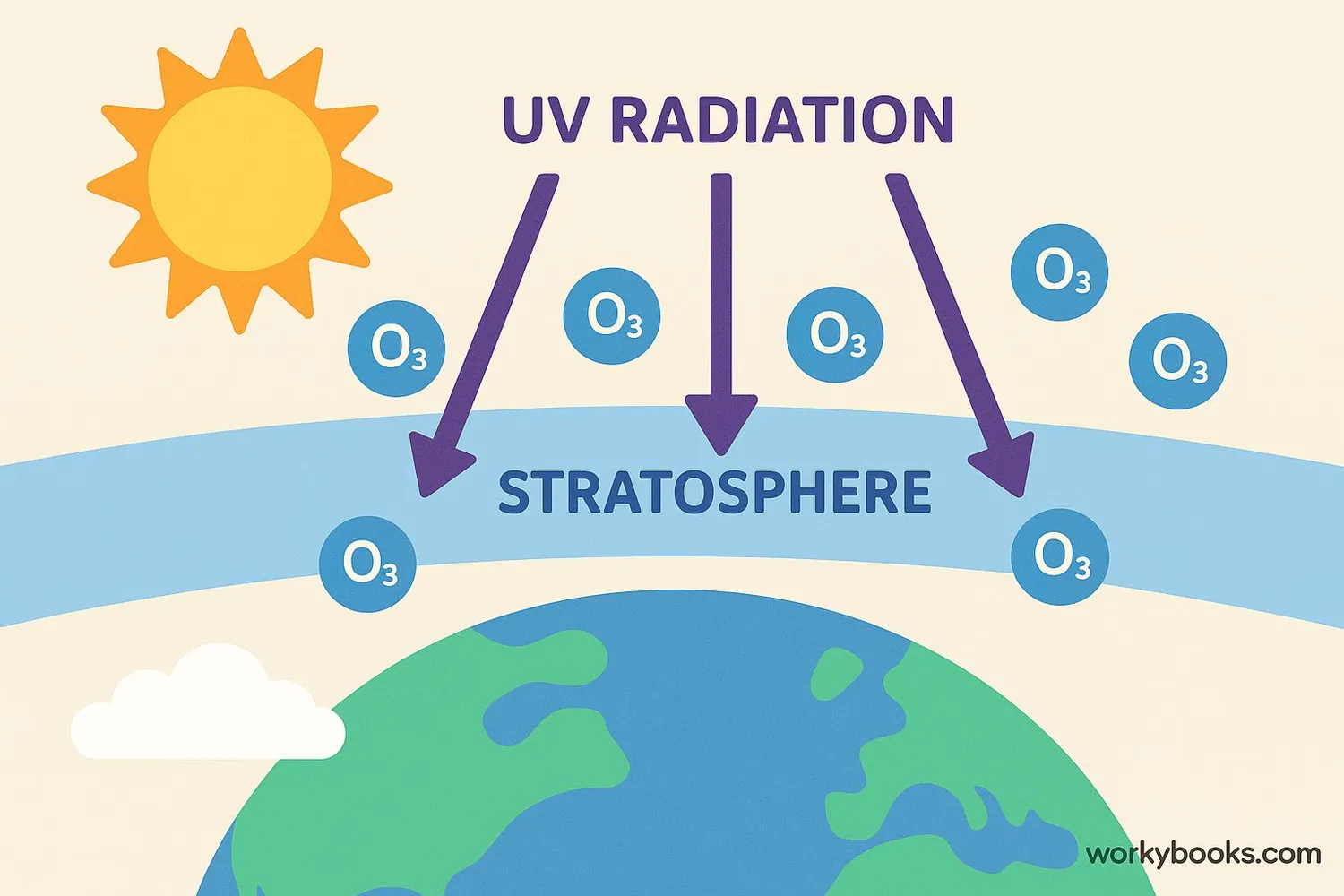
The ozone layer works like a natural filter for sunlight. Here's how it protects life on Earth:
Sun Emits UV Rays
The sun sends out different types of radiation, including harmful UVB rays
Ozone Absorption
Ozone molecules in the stratosphere absorb UVB radiation
Energy Conversion
UV energy is converted into harmless heat energy
Protection
Only safe amounts of UV radiation reach Earth's surface
This protection is vital because too much UV radiation can cause sunburns, skin cancer, eye damage, and harm plants and animals. The ozone layer blocks about 97-99% of the sun's medium-frequency ultraviolet light (UVB).
Example
Just like wearing sunglasses protects your eyes from bright sunlight, the ozone layer protects Earth from dangerous UV rays!
Ozone Depletion
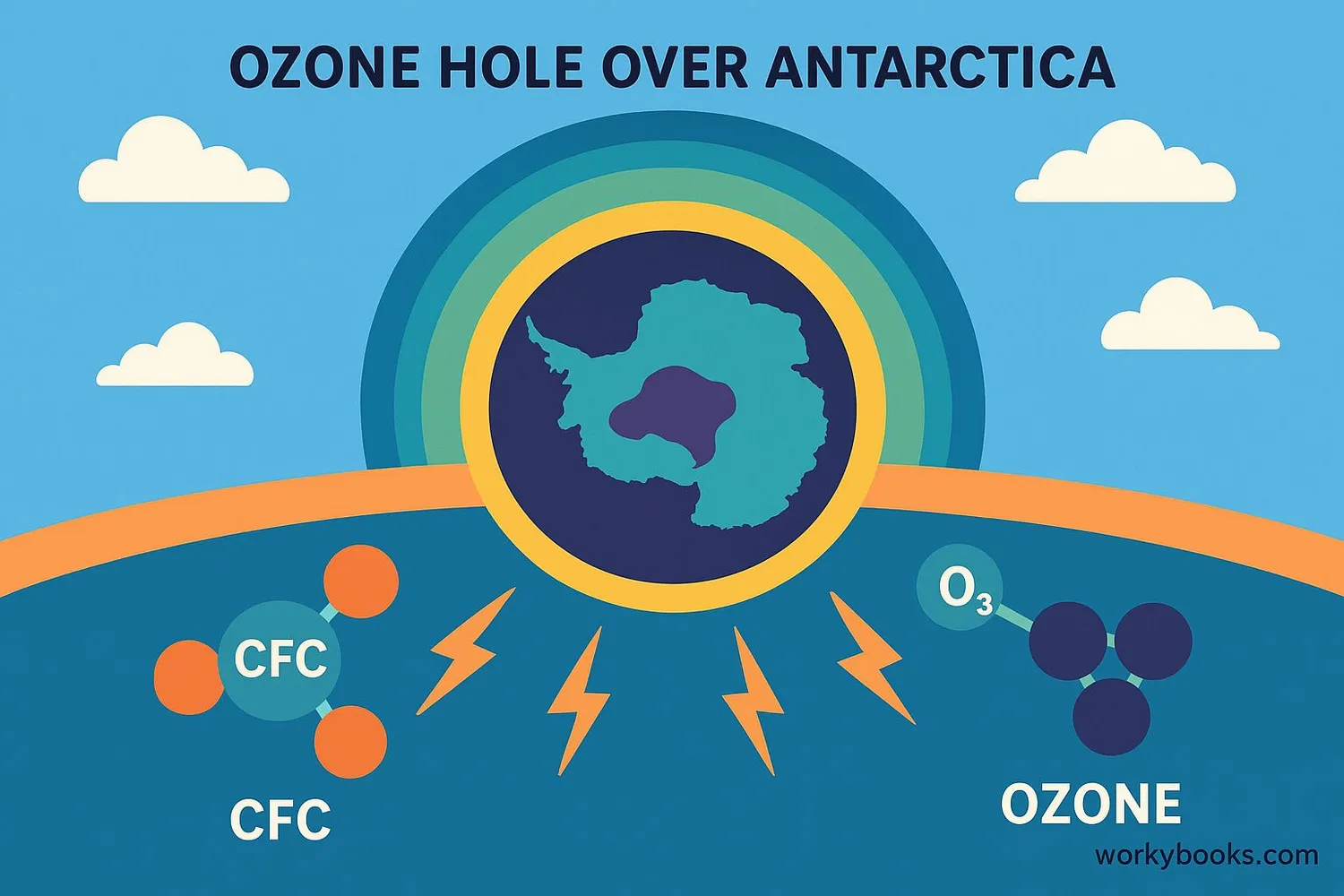
In the 1970s, scientists discovered that the ozone layer was getting thinner, especially over Antarctica where an "ozone hole" forms each spring. This depletion is primarily caused by human-made chemicals called ozone depleting substances (ODS), especially chlorofluorocarbons (CFCs).
How ozone depletion happens:
• CFCs rise to the stratosphere
• Sunlight breaks down CFCs releasing chlorine atoms
• Chlorine atoms destroy ozone molecules
• One chlorine atom can destroy 100,000 ozone molecules!
Historical Fact
The ozone hole was first discovered in 1985 by British scientists at the Halley Bay research station in Antarctica.
Recovery Efforts

The good news is that the world came together to address ozone depletion! In 1987, countries signed the Montreal Protocol, an international agreement to phase out ozone-depleting substances.
Thanks to this global cooperation:
• Production of CFCs has been almost eliminated worldwide
• Scientists have observed the ozone layer beginning to recover
• The ozone hole is expected to heal completely by 2060s
This shows that when countries work together, we can solve global environmental problems!
Global Agreement
197 countries signed the Montreal Protocol
CFC Phase-out
Production of CFCs reduced by over 99%
Ozone Recovery
Ozone layer is healing at 1-3% per decade
Ozone Layer Quiz
Test your knowledge about the ozone layer with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about the ozone layer:
Ozone Layer Trivia
Discover some fascinating facts about the ozone layer!
Natural Sunscreen
Just one centimeter of pure ozone at Earth's surface would be enough to protect us from UV radiation - that's how powerful this natural shield is!
Unexpected Discovery
The ozone hole was discovered by accident in 1985 when scientists noticed their instruments recording surprisingly low ozone levels over Antarctica.
Global Success Story
The Montreal Protocol is considered the most successful international environmental agreement, with all 197 UN member countries signing it!
Nobel Prize Research
In 1995, Paul Crutzen, Mario Molina, and Sherwood Rowland won the Nobel Prize in Chemistry for their work on ozone depletion chemistry.





