Anodes - Definition, Examples, Quiz, FAQ, Trivia
Discover how anodes power our world through electrochemical reactions
What is an Anode?
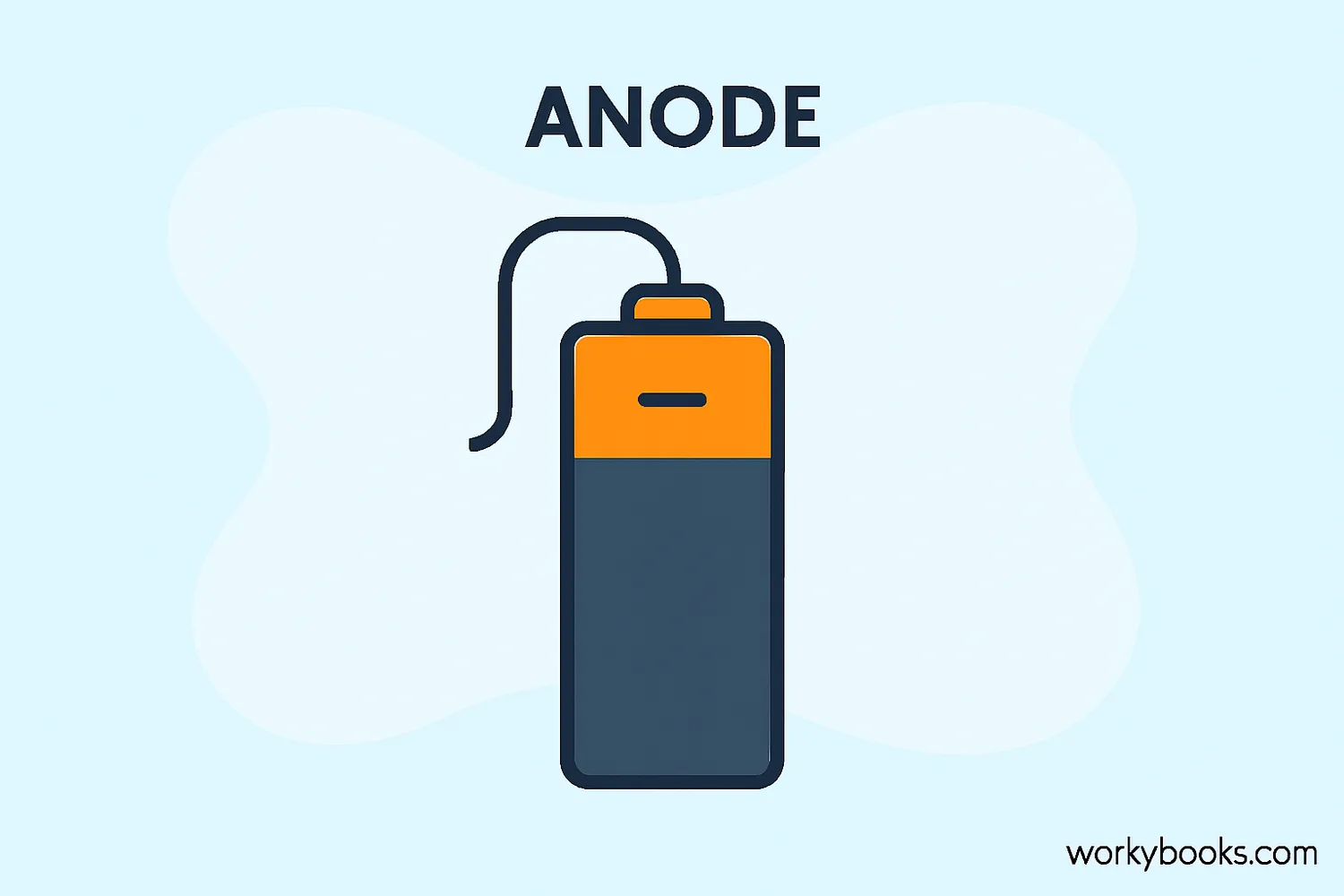
Anode Definition
An anode is the electrode where oxidation occurs in an electrochemical system. It's where electrons leave the system and electrical current enters.
Think of an anode as the "giving" end of a battery or circuit. When electricity flows, the anode:
• Releases electrons that travel through the circuit
• Experiences oxidation (loses electrons)
• Is labeled as the negative (-) terminal in batteries
• Attracts anions (negatively charged ions) in solutions
In simple terms: An anode is where electricity starts its journey in electrochemical devices like batteries and fuel cells.
Remember This!
Anode = Oxidation = Negative terminal in batteries
Negative Terminal
In most batteries, the anode is the negative terminal
Electron Source
Electrons flow out from the anode into the circuit
Oxidation Site
Chemical oxidation reactions happen at the anode
How Anodes Work
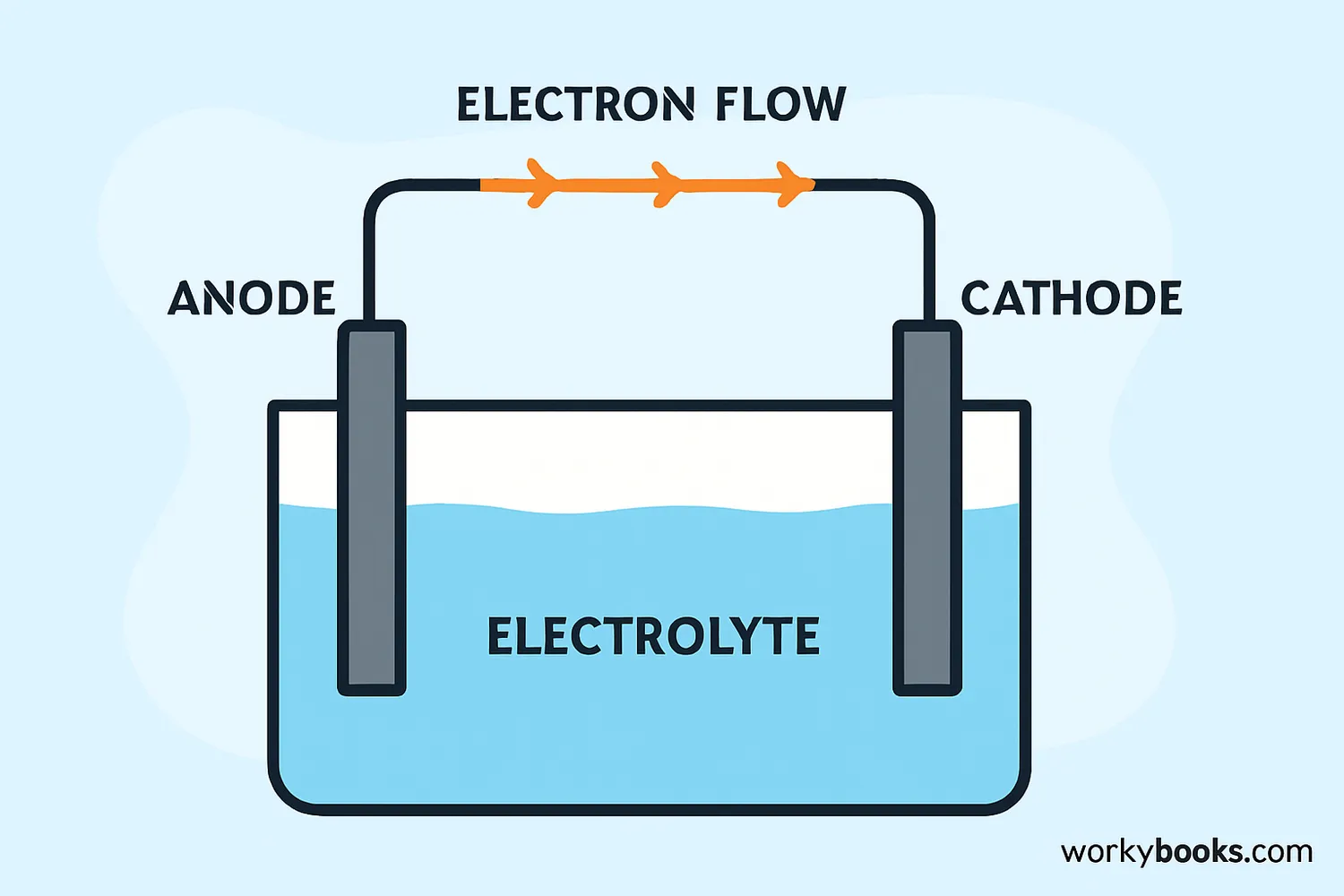
Anodes work through electrochemical reactions. Here's the step-by-step process:
Electron Release
Atoms at the anode surface lose electrons (oxidation)
Current Flow
Released electrons flow through the external circuit
Ion Movement
Positive ions move toward the cathode in solution
Reaction Completion
Electrons complete the circuit at the cathode
Key differences between anodes and cathodes:
| Feature | Anode | Cathode |
|---|---|---|
| Reaction Type | Oxidation | Reduction |
| Electron Flow | Electrons leave | Electrons enter |
| Battery Terminal | Negative (-) | Positive (+) |
Real-World Example
In a zinc-carbon battery, the zinc container serves as the anode and oxidizes to release electrons: Zn → Zn²⁺ + 2e⁻
Why Anodes Matter
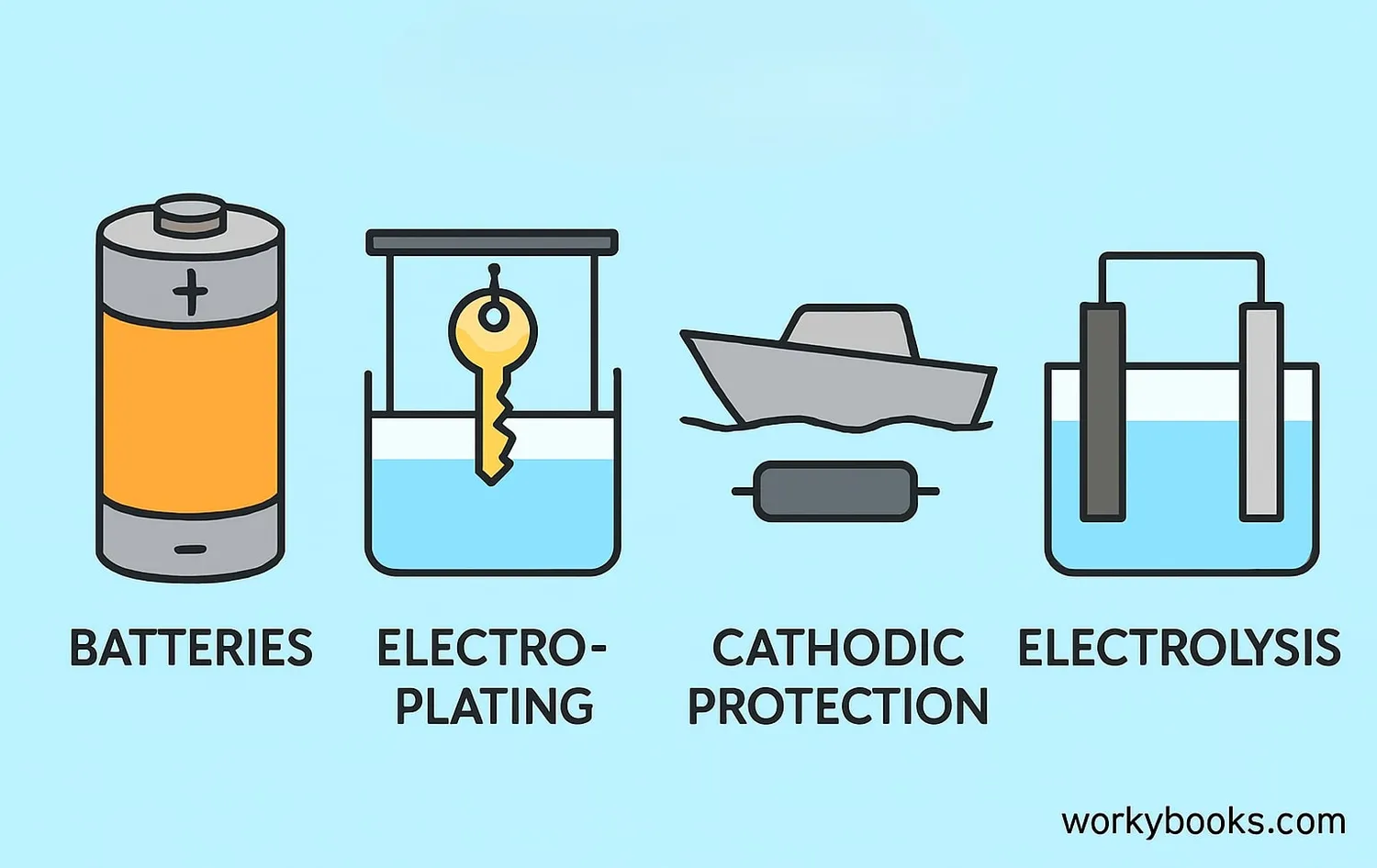
Anodes are essential components in many technologies we use every day:
Batteries
Power sources for devices from phones to electric cars
Electroplating
Coating objects with metal layers for protection or decoration
Corrosion Prevention
Sacrificial anodes protect ships and pipelines from rust
Special types of anodes:
• Sacrificial anodes: Made of reactive metals (like zinc or magnesium) that corrode instead of protected structures
• Inert anodes: Don't participate in reactions (like graphite in electrolysis)
• Consumable anodes: Provide metal ions for processes like electroplating
Without anodes, we wouldn't have portable electronics, electric vehicles, or many industrial processes!
Did You Know?
The largest sacrificial anode ever made weighed over 4 tons and protects offshore oil platforms!
Anode Knowledge Quiz
Test your understanding of anodes with this interactive quiz. Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about anodes:
Electrochemistry Trivia
Discover fascinating facts about anodes and electrochemistry:
Historical Origins
The term "anode" comes from Greek words "ana" (up) and "hodos" (way), meaning "the way upward." It was coined by scientist Michael Faraday in 1834.
Giant Anodes
The anodes used in aluminum production can weigh over 1,000 kg each! Aluminum smelters use thousands of these carbon anodes simultaneously.
Ship Protection
Large ships use sacrificial zinc anodes to prevent corrosion. A single cargo ship might have over 100 zinc anodes, each weighing 20-30 kg!
Lightning Fast Reactions
In fuel cells, electrochemical reactions at the anode can happen in nanoseconds. This allows fuel cells to respond quickly to power demands.





