Chemical Changes - Definition, Examples, Quiz, FAQ, Trivia
Discover how substances transform into something new!
What is Chemical Change?
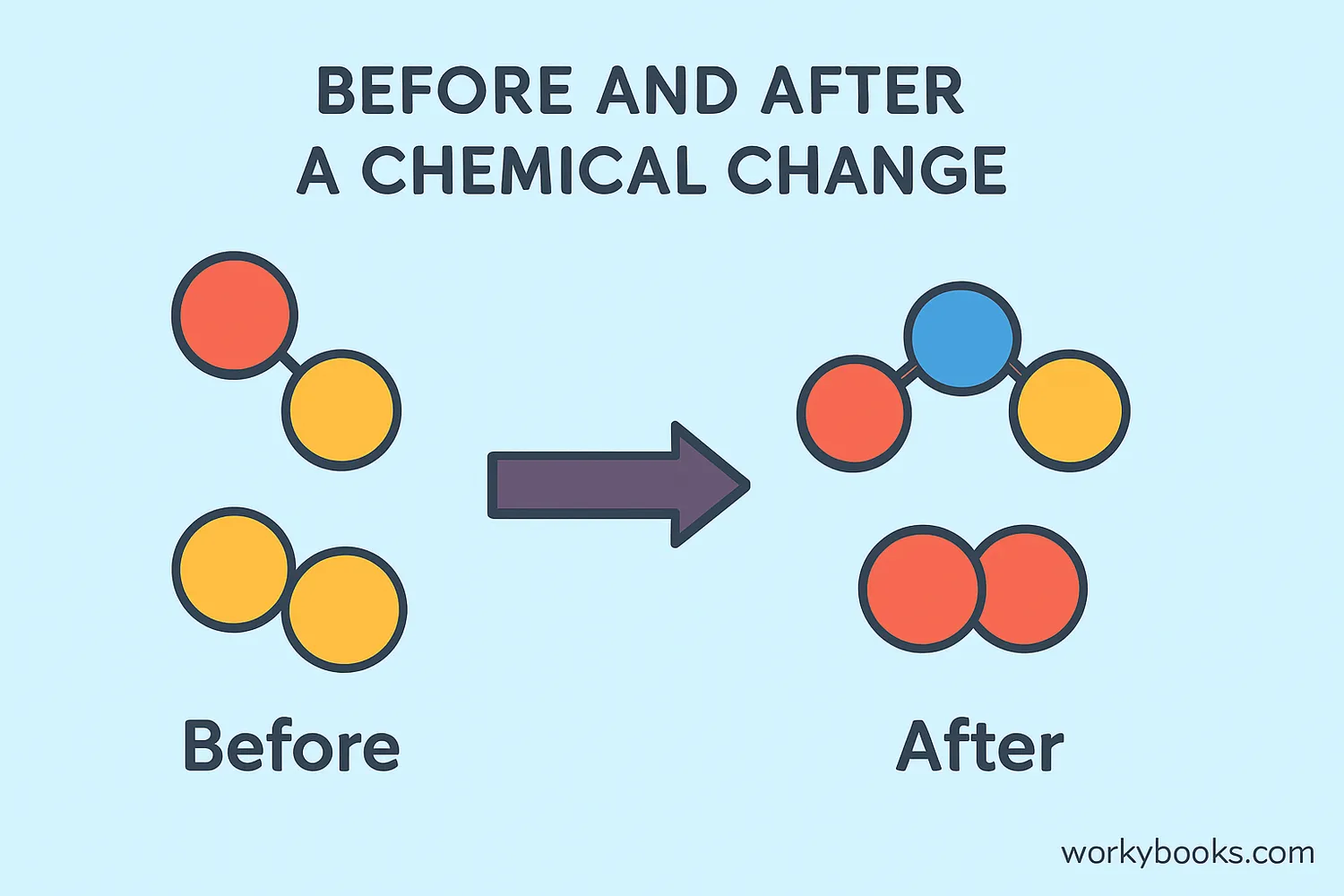
A chemical change happens when substances transform into completely new materials! It's like a molecular magic trick where atoms rearrange themselves to form new substances with different properties.
During a chemical change, the original substances disappear and new ones appear. This change is usually permanent - you can't get the original materials back easily. Think of baking a cake: once you've mixed the ingredients and baked them, you can't separate them back into flour, eggs, and sugar!
Key Fact
Chemical changes create new substances with different properties from the original materials.
Physical vs Chemical Changes
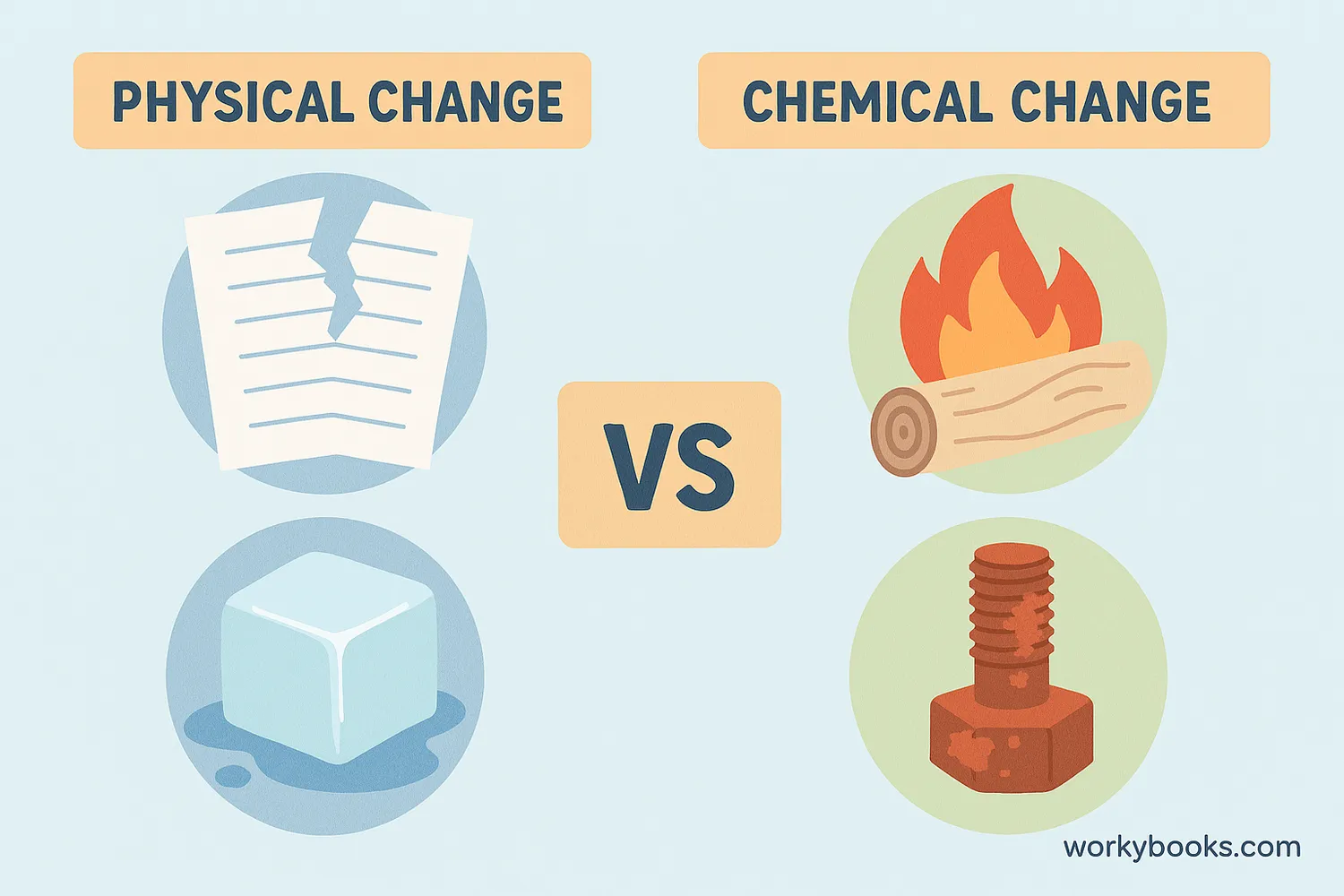
Understanding the difference between physical and chemical changes is important:
Physical Changes
Change shape or state but not identity (melting ice, tearing paper)
Chemical Changes
Form new substances (burning wood, rusting metal)
Key Differences:
• Physical changes are usually reversible
• Chemical changes create new substances
• Physical changes don't change chemical composition
• Chemical changes involve energy changes
For example, freezing water into ice is physical - it's still water. But baking a cake is chemical - the ingredients transform into something new!
Remember This
If you can reverse the change easily, it's probably physical. If new substances are formed, it's chemical!
Signs of Chemical Change
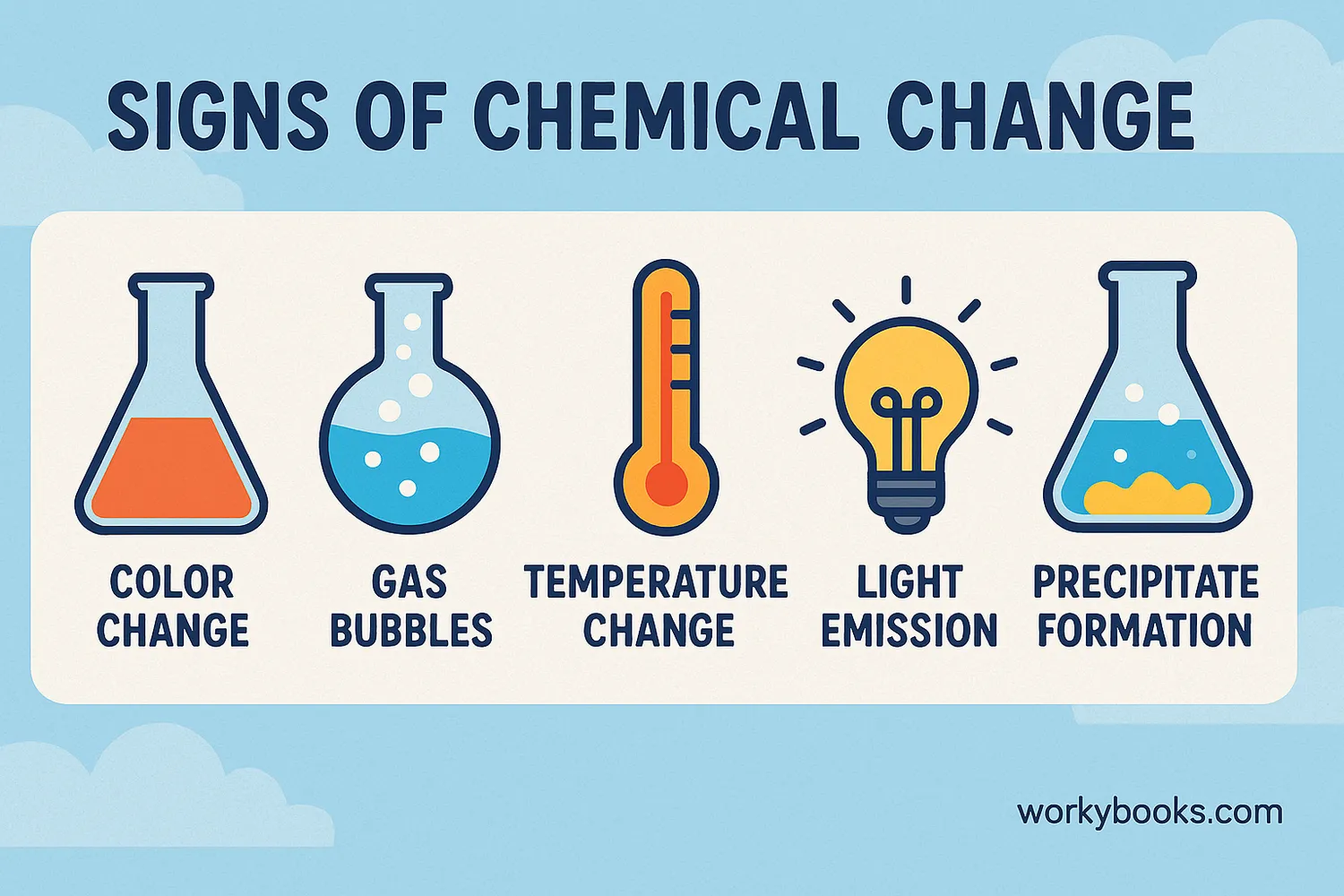
How can you tell when a chemical change is happening? Look for these clues:
Color Change
When substances change color unexpectedly
Gas Production
Bubbles or fizzing appear
Temperature Change
Heat is released (exothermic) or absorbed (endothermic)
Light Emission
Light is produced (like in fireworks)
Precipitate Forms
A solid appears when two liquids mix
Remember: These signs don't guarantee a chemical change has occurred, but they're good indicators. For example, changing the color of water with food coloring is physical, but iron rusting and turning reddish-brown is chemical!
Examples of Chemical Changes
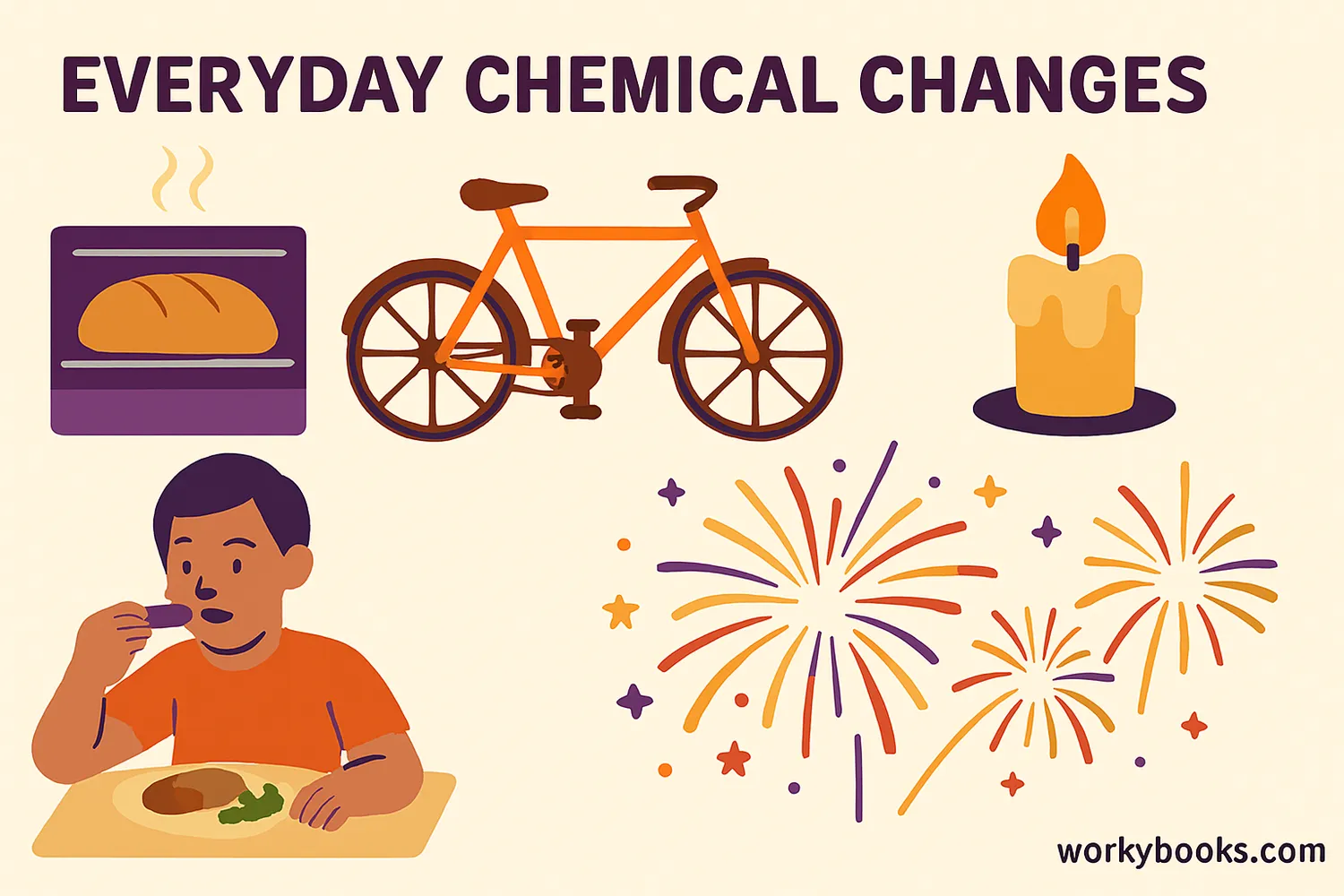
Chemical changes are all around us! Here are common examples you encounter every day:
Cooking & Baking
Ingredients transform into new foods (baking cake, frying eggs)
Burning
Wood turns to ash and smoke when burned
Rusting
Iron reacts with oxygen to form rust
Digestion
Food breaks down chemically in your body
Batteries
Chemical reactions produce electricity
Is burning wood a chemical change? Yes! Wood turns into ash, smoke, and gases - completely new substances.
Is rusting a chemical change? Definitely! Iron combines with oxygen to form iron oxide (rust), which has different properties.
Chemical Changes Quiz
Test your knowledge about chemical changes with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about chemical changes:
Fun Chemical Change Trivia
Discover amazing facts about chemical changes:
Colorful Explosions
Fireworks get their colors from chemical changes! Different metal compounds produce different colors when heated: strontium = red, barium = green, copper = blue, sodium = yellow.
Apple Browning
When apples turn brown after cutting, that's a chemical change! Oxygen in the air reacts with enzymes in the apple to form new brown-colored compounds.
Statue Transformations
The Statue of Liberty is green because of chemical change! Its copper surface reacted with oxygen and water to form a green patina called verdigris over 30 years.
Baking Magic
Baking soda causes cakes to rise through chemical change! When mixed with acid (like vinegar or lemon juice), it produces carbon dioxide gas that makes bubbles in batter.





