Chemical Formula - Definition, Examples, Quiz, FAQ, Trivia
Discover the language of chemistry and how chemical formulas help us understand the world around us!
What is a Chemical Formula?
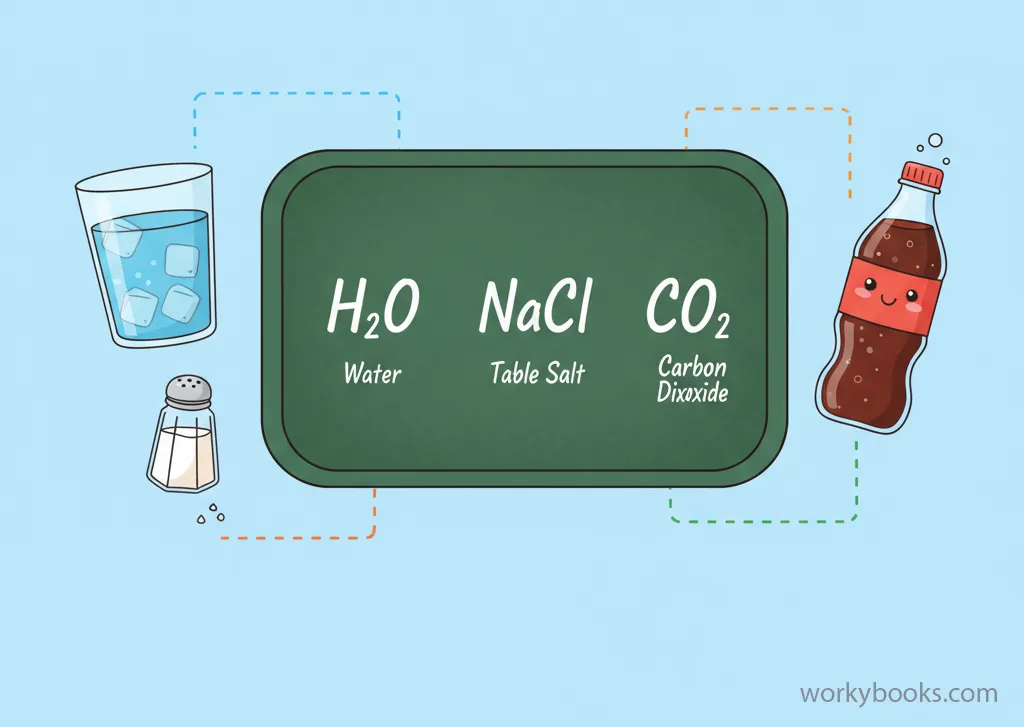
A chemical formula is like a special code that scientists use to represent a chemical compound. It tells us which elements are in the compound and how many atoms of each element are present.
Think of it as a recipe that shows what ingredients (elements) and how much of each ingredient (number of atoms) are needed to make a specific substance.
Science Fact!
The water you drink every day has a chemical formula: H₂O. This means each water molecule is made of 2 hydrogen atoms and 1 oxygen atom.
Chemical formulas use symbols from the periodic table to represent elements, and numbers (called subscripts) to show how many atoms of each element are in one molecule of the compound.
Chemical Symbols and Elements
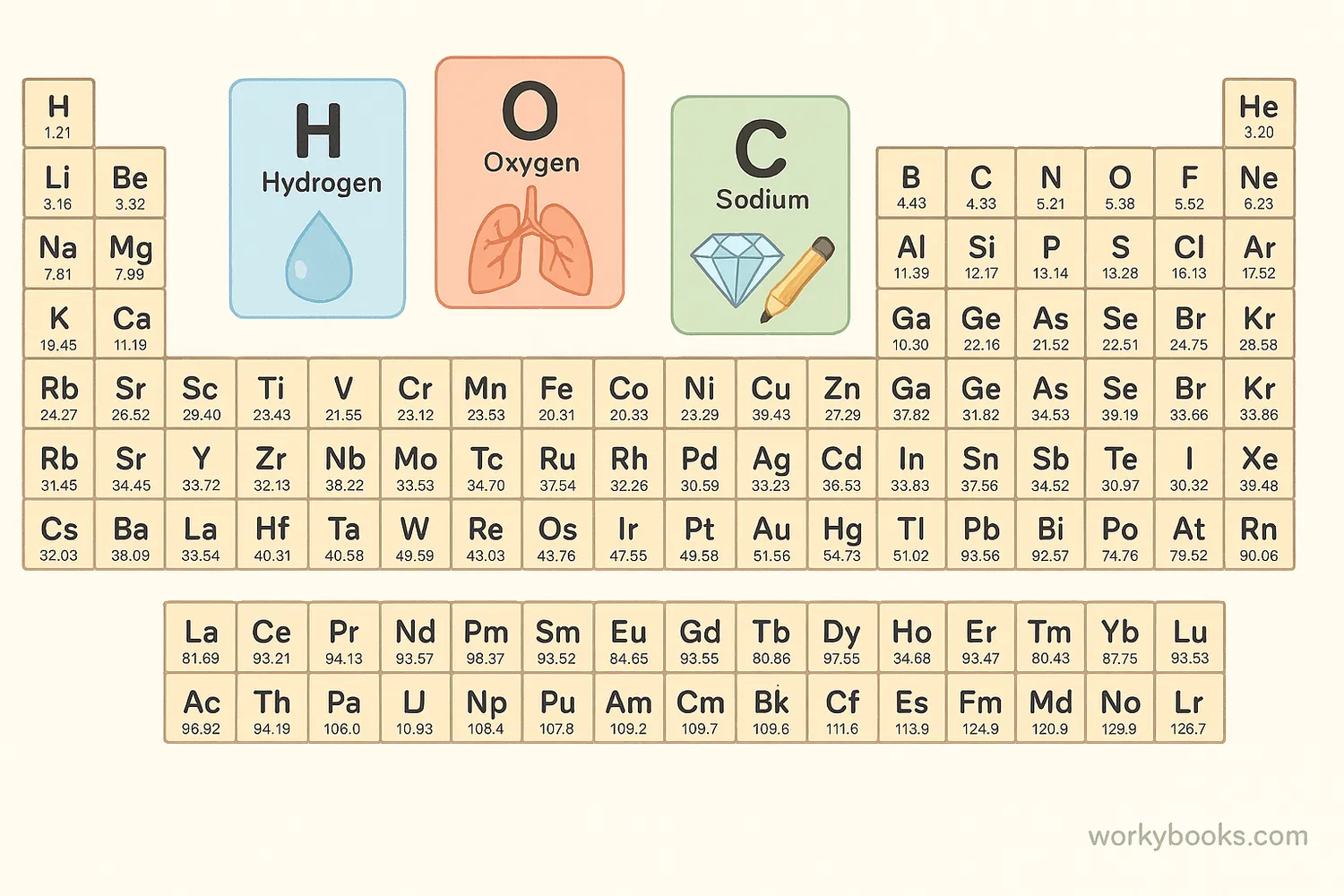
Before we can understand chemical formulas, we need to know about chemical symbols. These are one or two-letter abbreviations for chemical elements. Each element has its own unique symbol.
Common Element Symbols
Here are some common elements and their symbols that you might recognize:
| Element Name | Symbol | Common Uses |
|---|---|---|
| Hydrogen | H | Water, stars, fuel |
| Oxygen | O | Air we breathe, water |
| Carbon | C | All living things, diamonds |
| Nitrogen | N | Most of Earth's atmosphere |
| Sodium | Na | Table salt, nerves |
| Chlorine | Cl | Table salt, water purification |
Some symbols don't match the element's English name because they come from Latin or other languages. For example, sodium's symbol is Na from the Latin word "natrium."
Writing Chemical Formulas
Writing chemical formulas follows specific rules. Let's learn the basic steps:
Identify Elements
Determine which elements are in the compound
Write Symbols
Write the symbols for each element
Add Numbers
Add subscript numbers to show atom counts
Simplify
Write the formula in its simplest form
Rules for Writing Formulas
- The element that comes first in the name usually comes first in the formula
- Subscript numbers come after the element symbol
- If there's only one atom of an element, no subscript is needed
- Some formulas have parentheses with subscripts outside
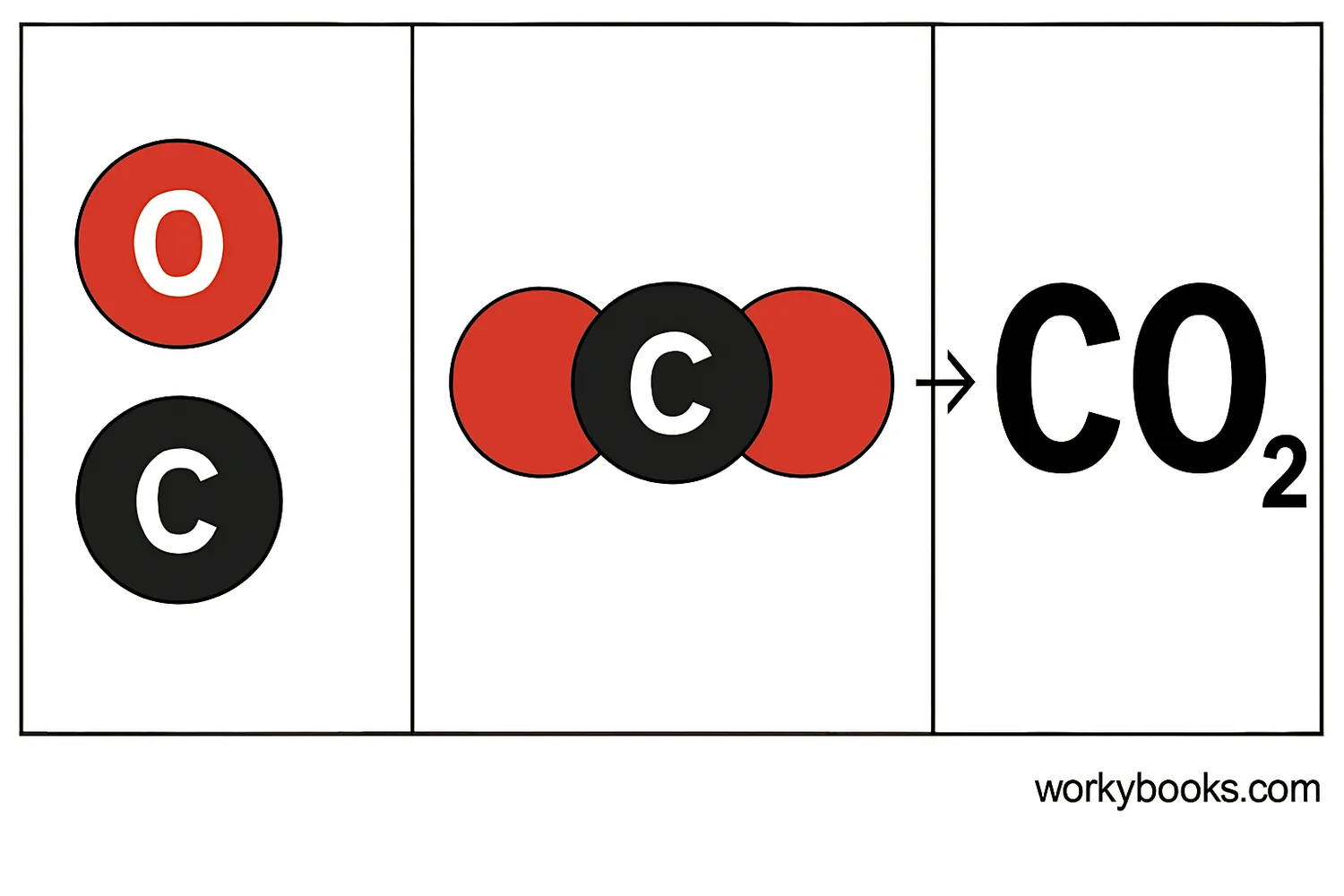
Example: Carbon Dioxide
Carbon dioxide has 1 carbon atom and 2 oxygen atoms. Its formula is written as CO₂.
The subscript "2" tells us there are 2 oxygen atoms. Since there's only 1 carbon atom, we don't write a subscript for carbon.
Common Chemical Formulas
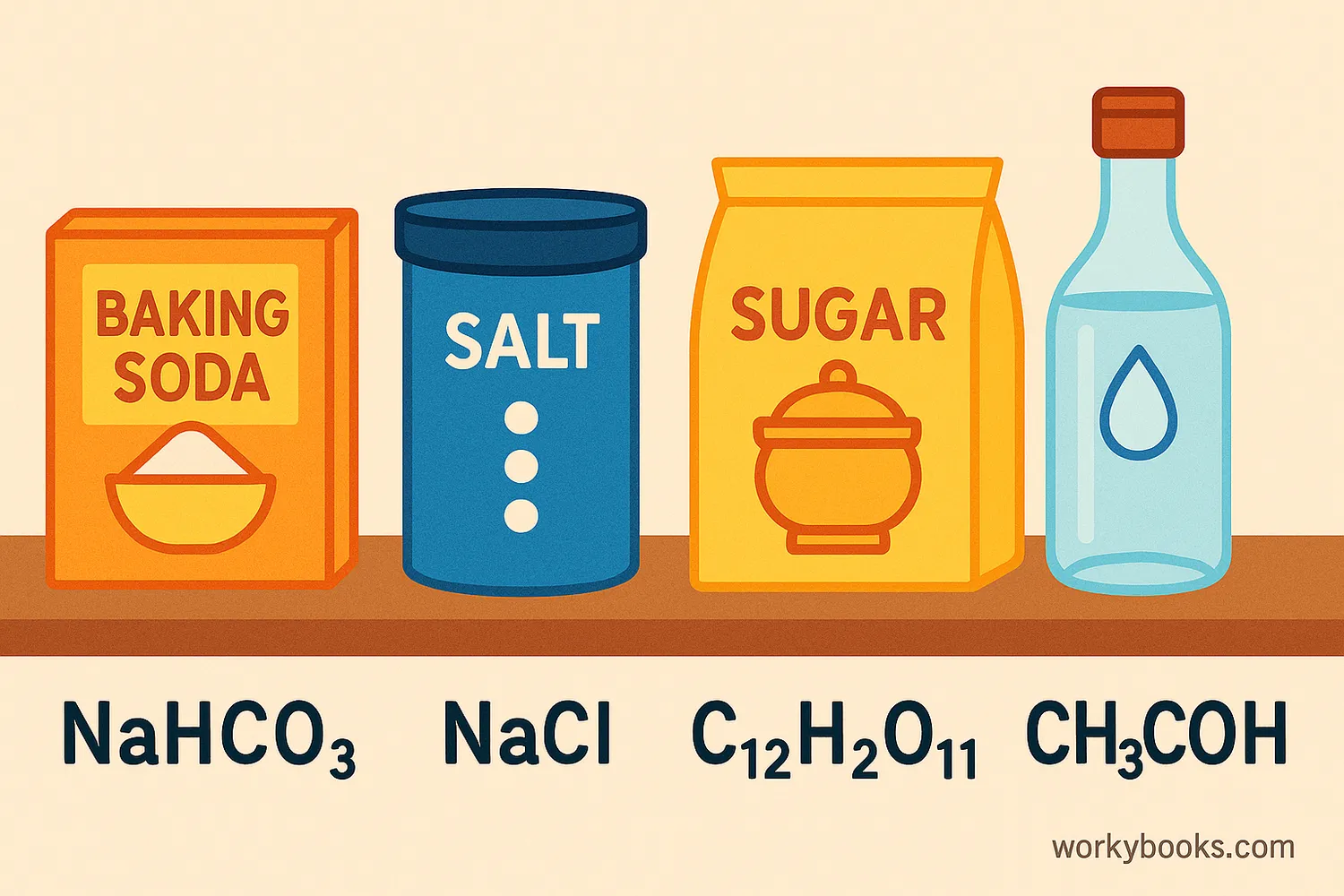
You might be surprised to learn that many everyday substances have chemical formulas. Here are some common ones:
| Substance | Chemical Formula | What It Means |
|---|---|---|
| Water | H2O | 2 hydrogen atoms, 1 oxygen atom |
| Table Salt | NaCl | 1 sodium atom, 1 chlorine atom |
| Carbon Dioxide | CO2 | 1 carbon atom, 2 oxygen atoms |
| Sugar | C12H22O11 | 12 carbon, 22 hydrogen, 11 oxygen atoms |
| Baking Soda | NaHCO3 | 1 sodium, 1 hydrogen, 1 carbon, 3 oxygen atoms |
| Vinegar | CH3COOH | 2 carbon, 4 hydrogen, 2 oxygen atoms |
Notice how some formulas like NaCl don't have any subscript numbers. This means there's one atom of each element in the compound.
Remember!
The numbers in chemical formulas are very important. For example, CO (carbon monoxide) is a poisonous gas, while CO₂ (carbon dioxide) is what we exhale and plants use for photosynthesis. Just one oxygen atom makes a big difference!
Chemical Equations
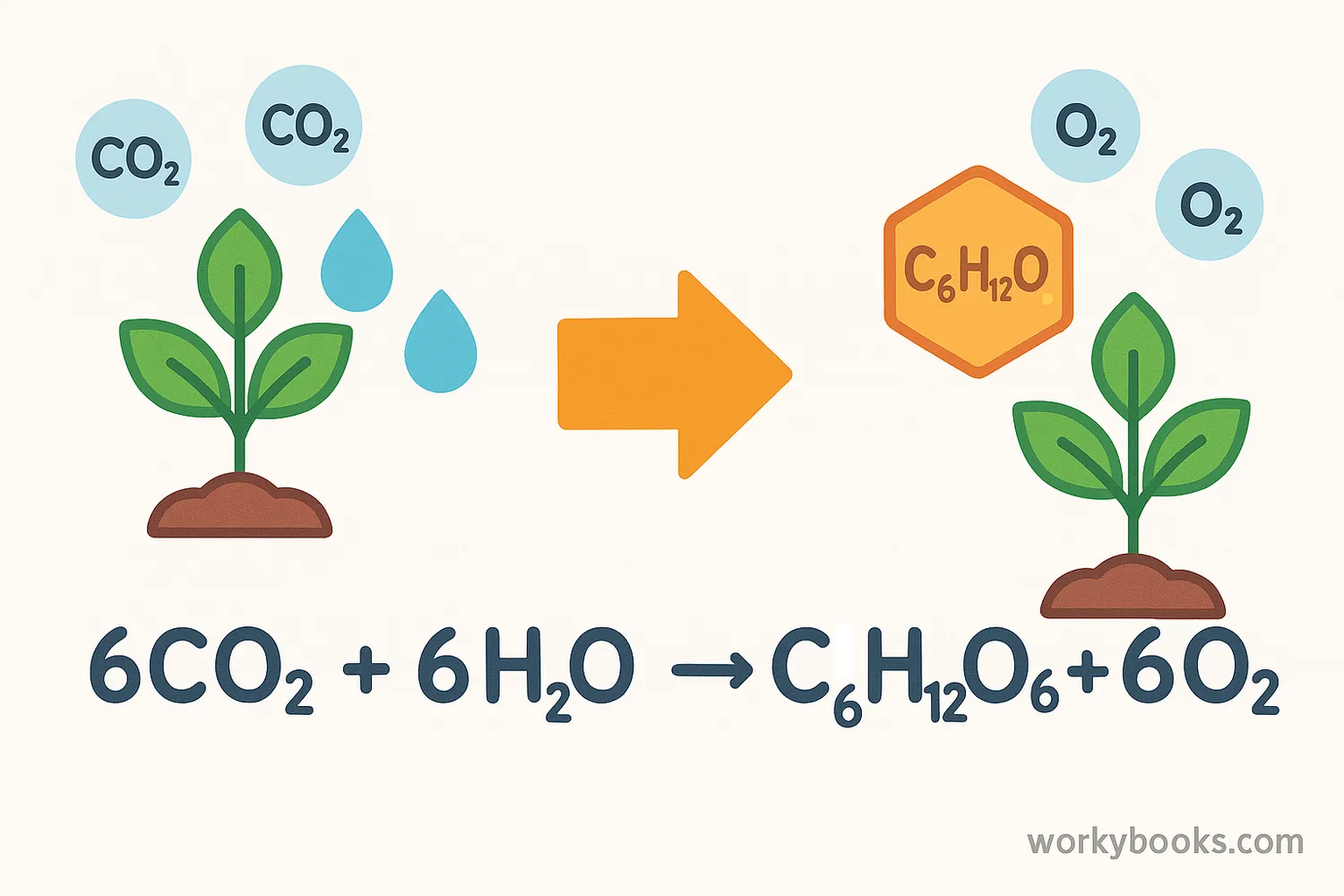
Chemical formulas are used to write chemical equations, which show how substances change during chemical reactions. A chemical equation has reactants (what you start with) on the left and products (what you end up with) on the right, separated by an arrow.
This equation shows that two molecules of hydrogen gas (H₂) combine with one molecule of oxygen gas (O₂) to form two molecules of water (H₂O).
Arrow
Means "yields" or "produces"
Plus Sign
Separates different reactants or products
State Symbols
Show physical state: (s)=solid, (l)=liquid, (g)=gas, (aq)=dissolved in water
Coefficients
Numbers in front that multiply the entire formula
Law of Conservation of Mass
In chemical reactions, matter is neither created nor destroyed. This means the number of atoms of each element must be the same on both sides of the equation. This is called balancing equations.
Chemistry Quiz
Test your understanding of chemical formulas with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about chemical formulas:
Science Trivia
Discover some amazing facts about chemical formulas!
Ancient Symbols
Before modern chemical symbols were developed, alchemists used mysterious symbols to represent elements. For example, they used a crescent moon for silver, a circle with a dot for gold, and a triangle for water. These symbols were often kept secret!
Complex Formulas
Some chemical formulas are incredibly complex. The formula for chlorophyll, the molecule that makes plants green, is C₅₅H₇₂MgN₄O₅. Even more complex is the formula for DNA, which would be incredibly long if written out completely because DNA contains millions of atoms!
Formulas in Space
Chemical formulas aren't just for Earth! Scientists have detected complex molecules in space using telescopes. For example, they've found sugar (glycolaldehyde, C₂H₄O₂) in gas clouds where new stars are forming, suggesting that the ingredients for life might be common throughout the universe.
Everyday Formulas
You encounter chemical formulas every day without realizing it! The screen you're looking at right now contains compounds with specific formulas. The air you breathe is mostly N₂ (nitrogen gas) and O₂ (oxygen gas). Even your body is made of compounds with specific formulas like water (H₂O) and DNA.





