Coulomb's Law - Definition, Examples, Quiz, FAQ, Trivia
Discover how electric charges push and pull each other!
What is Coulomb's Law?
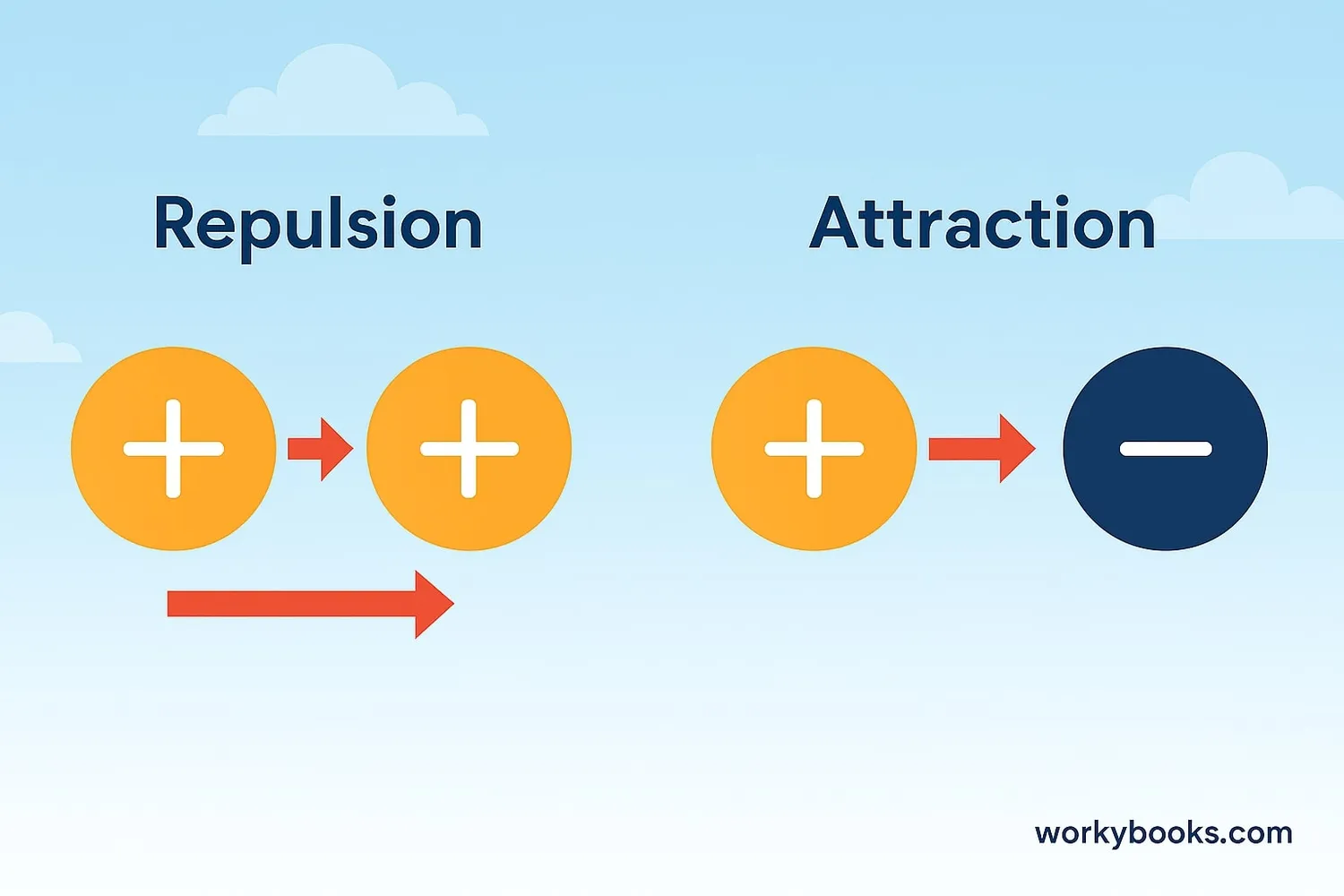
Coulomb's Law explains how electrically charged objects push or pull on each other. It was discovered by French scientist Charles-Augustin de Coulomb in 1785.
Think of it like magnets - similar charges repel (push away) while opposite charges attract (pull together). The strength of this electric force depends on two things: how much charge the objects have, and how far apart they are.
This formula means:
• F = Force between charges
• k = Coulomb's constant (9 × 10⁹ N·m²/C²)
• q₁ and q₂ = Amounts of electric charge
• r = Distance between the charges
Electric Charge Fact!
The smallest unit of charge is carried by electrons and protons. One electron has about 1.6 × 10⁻¹⁹ coulombs of charge!
How Coulomb's Law Works
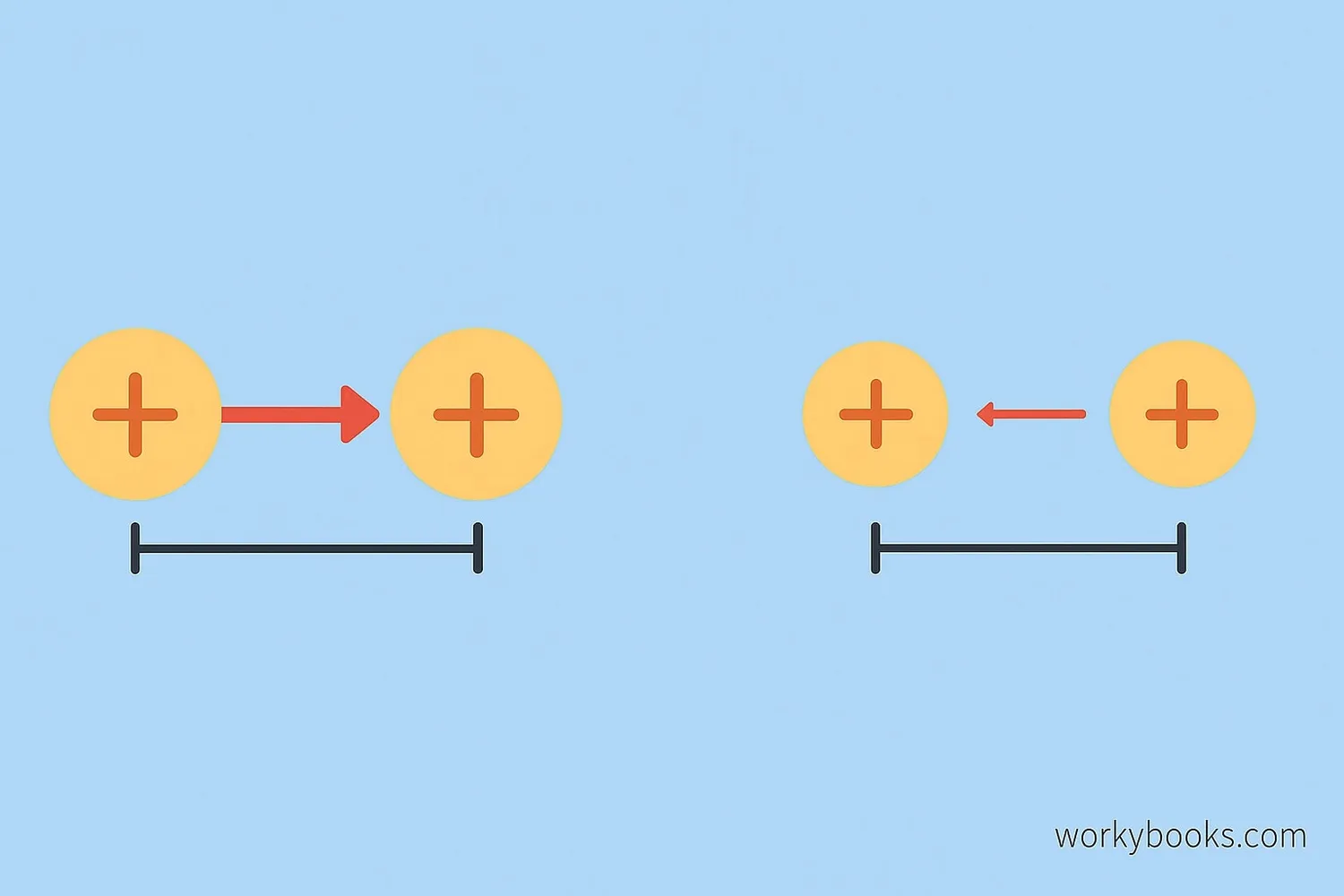
Coulomb's Law has two important rules that determine the strength of the electric force:
Charge Matters
More charge = stronger force. Double one charge = double the force.
Distance Matters
Force weakens quickly with distance. Double the distance = ¼ the force.
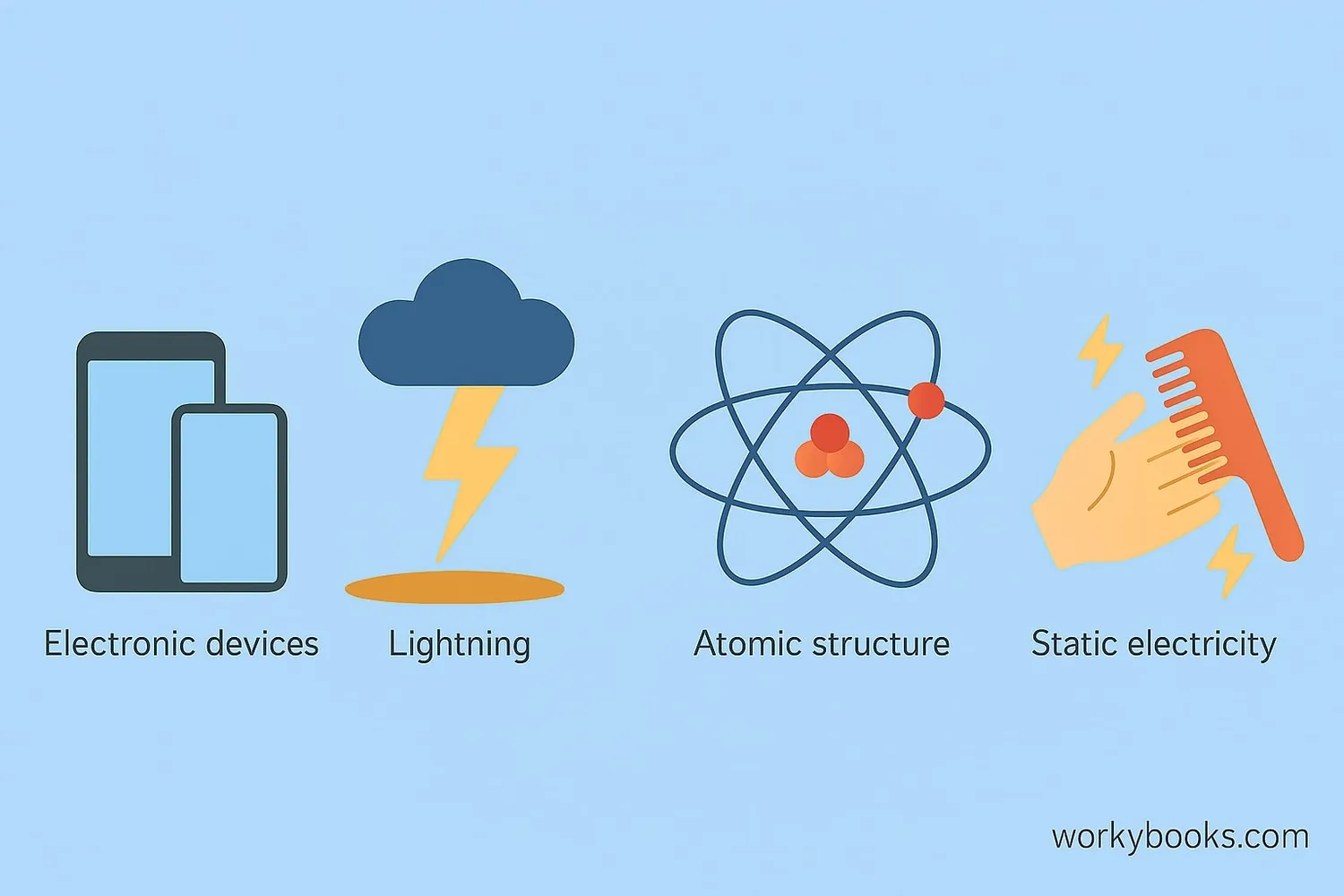
This law explains why:
• Your hair stands up when rubbed with a balloon
• Lightning jumps between clouds and the ground
• Static electricity makes clothes stick together
• Atoms hold electrons around their nucleus
Force Comparison
The electric force between two protons is about 10³⁶ times stronger than the gravitational force between them!
Why Coulomb's Law is Important
Coulomb's Law helps us understand and control electricity, which powers our modern world. Here's why it matters:
Electronics
Designing computers, phones, and all electronic devices
Energy Transfer
Understanding how electricity moves through wires
Atomic Structure
Explaining how electrons orbit atomic nuclei
Without understanding electric forces, we wouldn't have:
• Computers and smartphones
• Electric power grids
• Medical imaging devices
• Modern chemistry knowledge
• Understanding of lightning and weather
Electric Force Quiz
Test your knowledge of Coulomb's Law with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about Coulomb's Law:
Science Trivia
Discover amazing facts about electric forces:
Massive Power
The electric force between two 1-coulomb charges 1 meter apart would be 9 billion newtons - strong enough to lift 900 Eiffel Towers!
Electric Animals
Electric eels use Coulomb's Law to hunt! They generate up to 600 volts to stun prey. That's enough to power 100 light bulbs!
Atomic Glue
Coulomb's Law holds atoms together! The force between positive protons and negative electrons keeps electrons in orbit around the nucleus.
Scale Matters
At atomic scales, electric forces completely dominate gravity. The electric force between two electrons is 10⁴² times stronger than gravity!





