Distillation - Definition, Examples, Quiz, FAQ, Trivia
Discover how we separate mixtures using differences in boiling points
What is Distillation?
Distillation is a separation process that uses differences in boiling points to separate components of a liquid mixture. It's like when you boil saltwater - the water turns to steam and leaves the salt behind, then the steam cools down and becomes pure water again!
Think of distillation as a way to "sort" liquids based on how easily they evaporate. Different substances have different boiling points (the temperature at which they change from liquid to gas). By carefully controlling temperature, we can separate these substances from each other.
Science Fact!
Distillation is one of the oldest chemical processes, used for thousands of years to purify water and create perfumes!
How Distillation Works
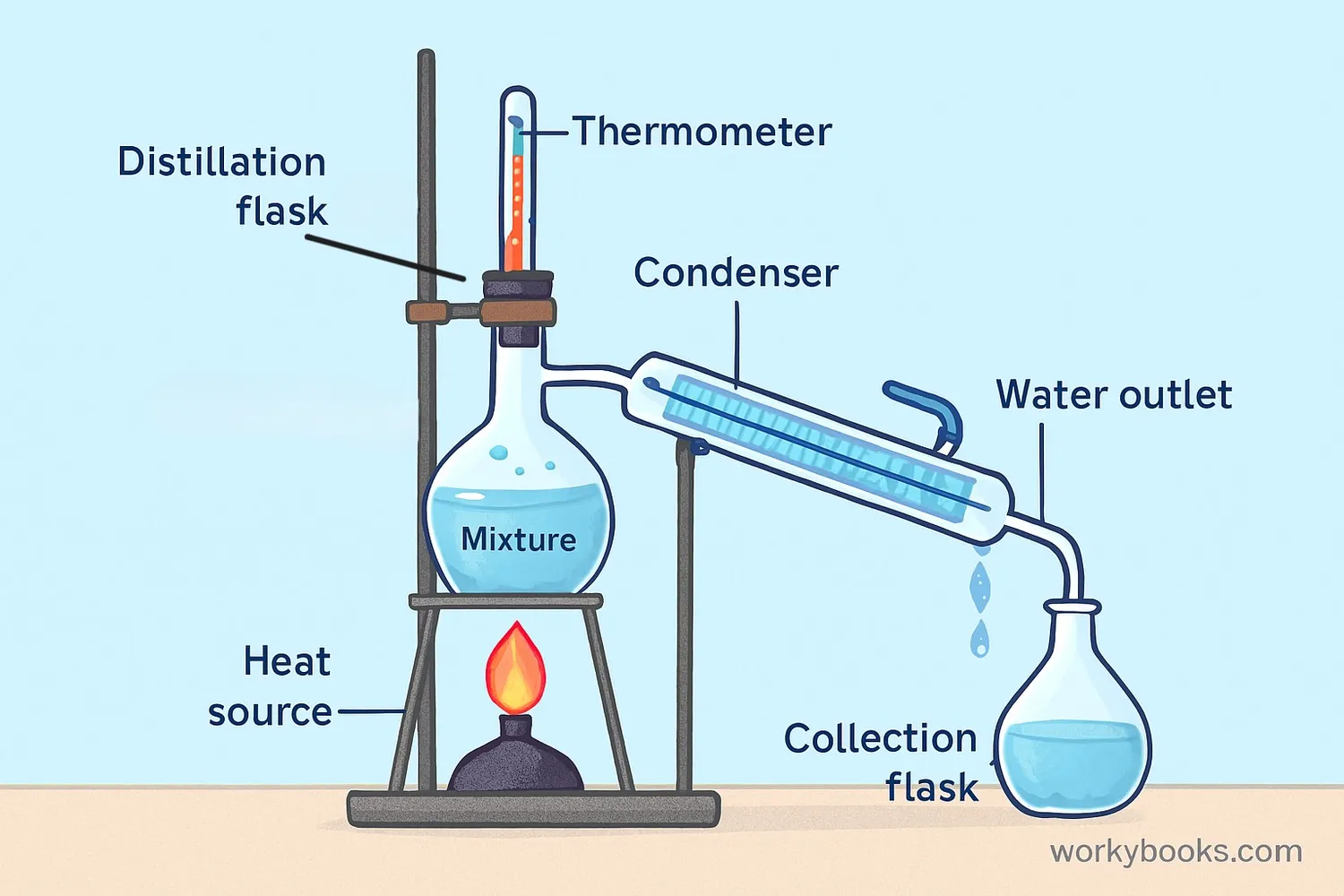
Distillation works through a cycle of vaporization and condensation. Here's how this amazing separation process works:
Heating
The mixture is heated in a special flask
Vaporization
The substance with the lower boiling point turns into vapor first
Cooling
The vapor travels to a cooled condenser tube
Condensation
The vapor cools and turns back into liquid
Collection
The purified liquid is collected in a separate container
The key principle is that different substances have different boiling points. For example, water boils at 100°C (212°F), while alcohol boils at around 78°C (172°F). By heating a mixture of water and alcohol to about 80°C, the alcohol will vaporize while most of the water stays liquid.
Boiling Point Knowledge!
Different liquids have different boiling points because of how strongly their molecules attract each other. The stronger the attraction, the more heat needed to separate them!
Types of Distillation
There are several types of distillation used for different purposes. Each method is specially designed for particular kinds of mixtures:
Simple Distillation
Used when the boiling points differ significantly. Good for separating salt from water.
Fractional Distillation
Uses a fractionating column for mixtures with closer boiling points, like in petroleum refining.
Steam Distillation
Used for temperature-sensitive compounds like essential oils from plants.
Vacuum Distillation
Lowers pressure to reduce boiling points for heat-sensitive materials.
Fractional distillation is especially important in industry. It uses a tall fractionating column with many trays or packing material. As vapors rise, they repeatedly condense and vaporize, becoming purer with each cycle. This is how crude oil is separated into gasoline, diesel, kerosene, and other products.
Uses and Importance of Distillation

Distillation is one of the most important separation processes in our world. It's used in many industries to purify chemicals and create products we use every day:
Petroleum Refining
Crude oil is separated into gasoline, diesel, and other products using fractional distillation
Alcohol Production
Distillation purifies alcoholic beverages and produces ethanol for fuel and industry
Water Purification
Desalination and purification of water for drinking and scientific use
Other important uses include:
• Production of medicines and pharmaceuticals
• Creation of essential oils and perfumes
• Chemical manufacturing and purification
• Recycling of solvents and chemicals
Without distillation, we wouldn't have many of the pure chemicals, fuels, and products that modern society depends on. It's a fundamental process that combines basic scientific principles with practical engineering to solve real-world problems.
Did You Know?
The world's largest distillation columns in oil refineries can be over 100 feet tall and separate thousands of barrels of crude oil daily!
Distillation Quiz
Test your distillation knowledge with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about distillation:
Interesting Distillation Facts
Discover some amazing facts about distillation!
Ancient Origins
Distillation was first used around 1200 BC by ancient civilizations to make perfumes and medicinal compounds. The earliest evidence comes from Mesopotamia and ancient India.
Massive Scale
The world's largest distillation column is in South Korea and stands over 100 meters tall! It can process hundreds of thousands of barrels of crude oil each day.
Alcohol Strength
Through repeated distillation, alcohol can be purified to very high concentrations. The strongest distilled spirits can reach up to 96% alcohol content!
Recycling Applications
Distillation is used to recycle many solvents and chemicals in industry. This helps reduce waste and conserve resources, making processes more environmentally friendly.





