Equilibrium Constant- Definition, Examples, Quiz, FAQ, Trivia
Discover how chemical reactions balance themselves and create equilibrium!
What is Chemical Equilibrium?
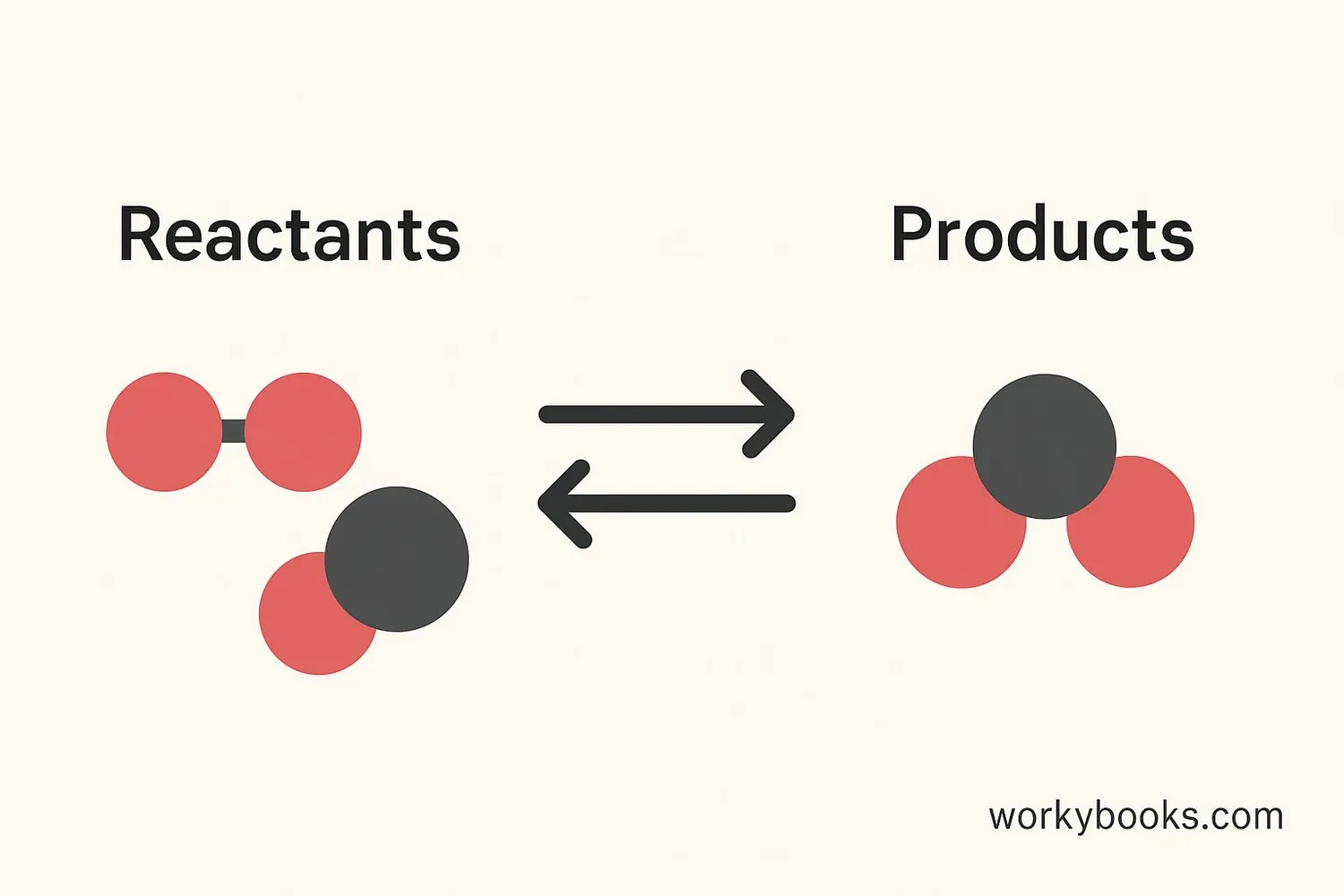
Chemical equilibrium is like a perfectly balanced seesaw! It's when a chemical reaction reaches a point where the forward reaction (reactants turning into products) happens at exactly the same rate as the reverse reaction (products turning back into reactants).
At equilibrium, the amounts of reactants and products don't change anymore, but both reactions are still happening. It's like two teams playing tug-of-war where both sides are equally strong - the rope doesn't move, but both teams are still pulling!
Science Fact!
Most chemical reactions in living things, like in your body, are at equilibrium!
The Equilibrium Constant (K)
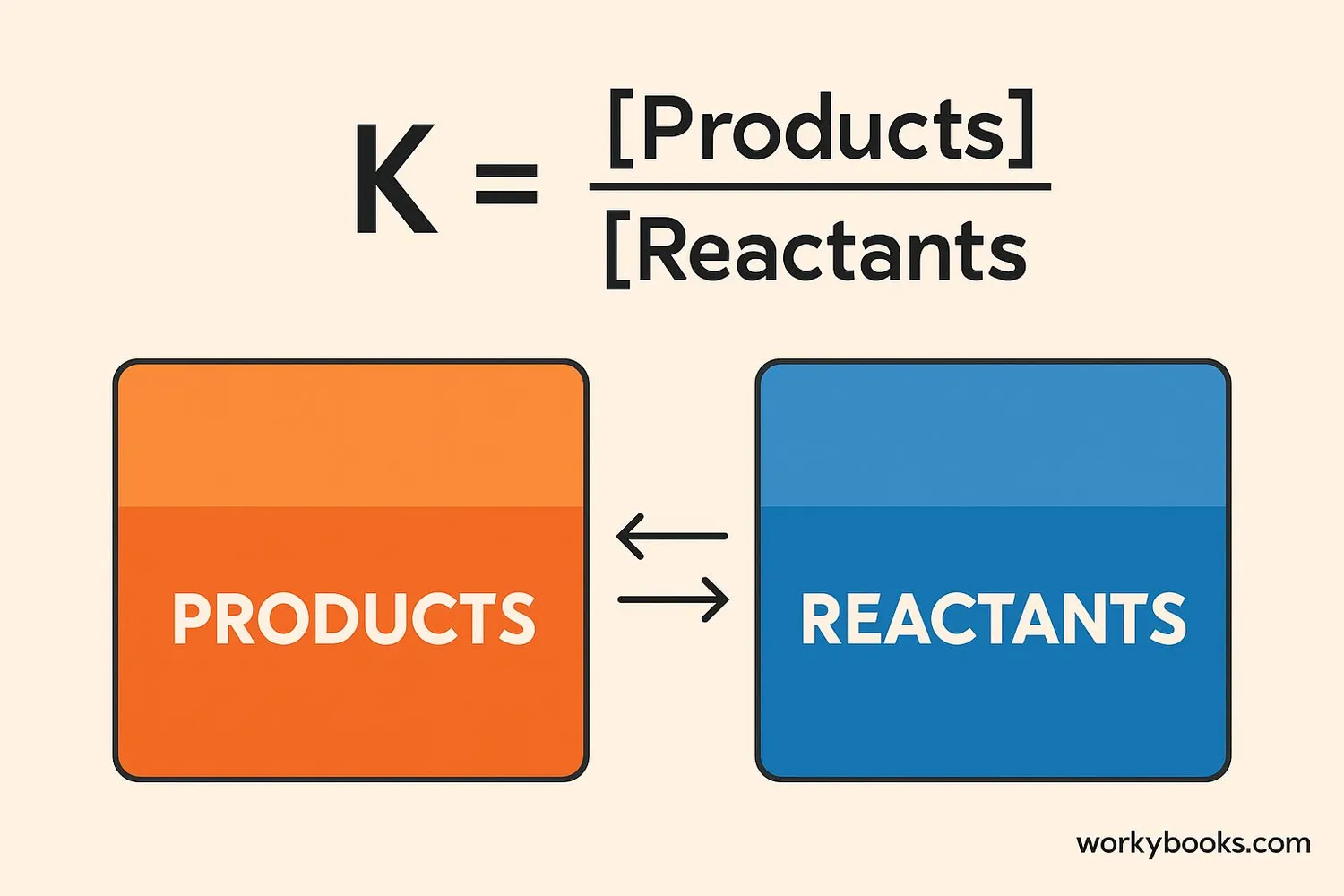
The equilibrium constant (K) is a special number that tells us how much products and reactants are present when a reaction reaches equilibrium. It's like a "balance score" for the reaction!
Scientists calculate K using this simple rule:
Here's what K tells us:
• If K is large (K > 1): More products than reactants at equilibrium
• If K is small (K < 1): More reactants than products at equilibrium
• If K = 1: Equal amounts of products and reactants
Law of Mass Action
The scientific rule that defines how K is calculated
Constant Value
K only changes with temperature
Reaction Direction
K tells us which way the reaction "prefers" to go
Important Note!
Equilibrium constants are always measured at a specific temperature. Change the temperature, and K changes!
Kc and Kp - Two Types of Constants
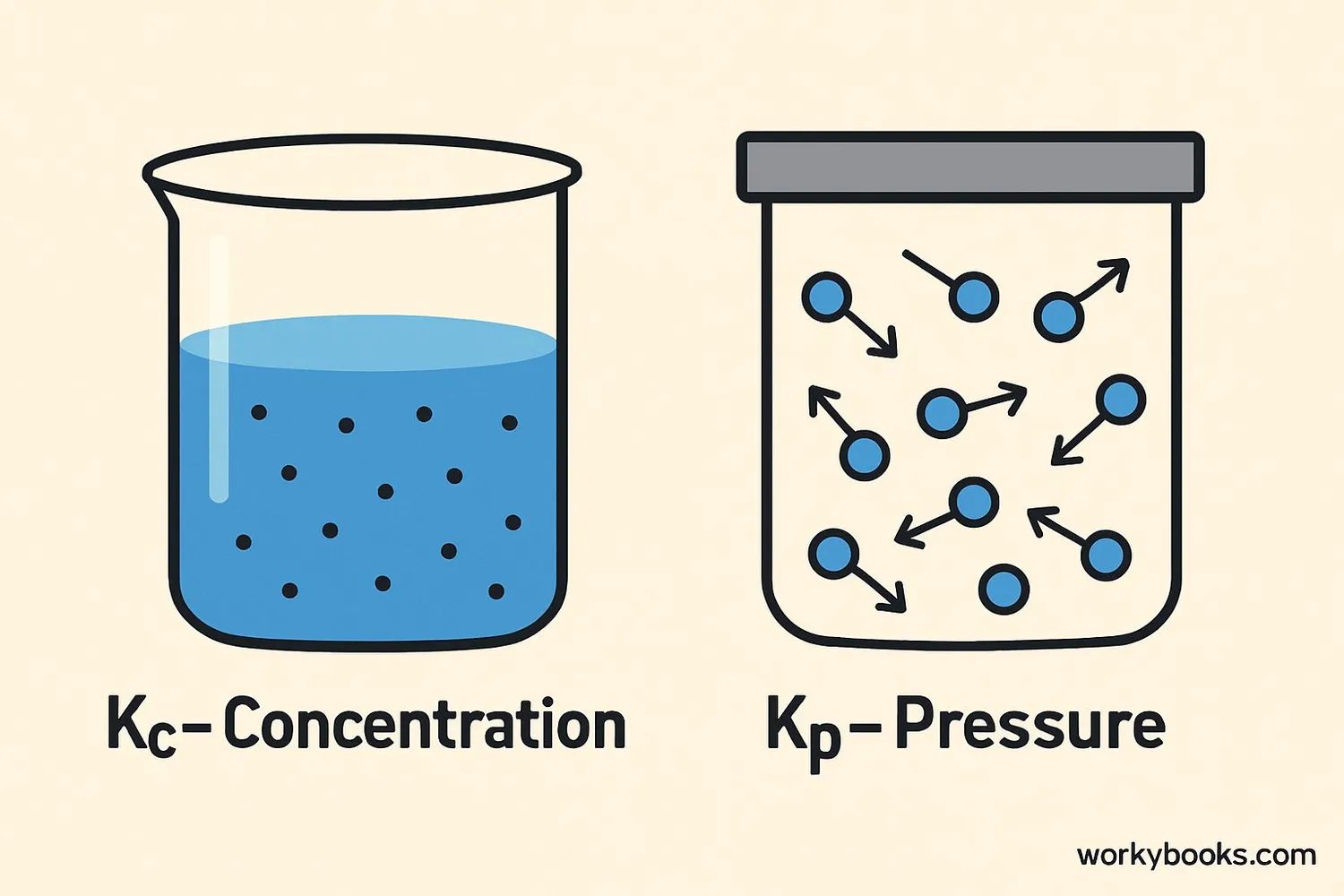
There are two main types of equilibrium constants:
Kc: This is used when we measure concentrations (how much substance is dissolved in a solution). For a reaction: aA + bB ⇌ cC + dD
Kp: This is used for gases, when we measure partial pressures instead of concentrations. For the same reaction:
Why two different constants? Because gases behave differently than substances in solution! Kc and Kp are related but not the same. They can be converted using temperature and the change in number of gas molecules.
Real World Example
When you open a soda bottle, you hear a "pssst" sound because CO₂ gas is escaping to reach a new equilibrium between the liquid and gas phases!
Reaction Quotient (Q)
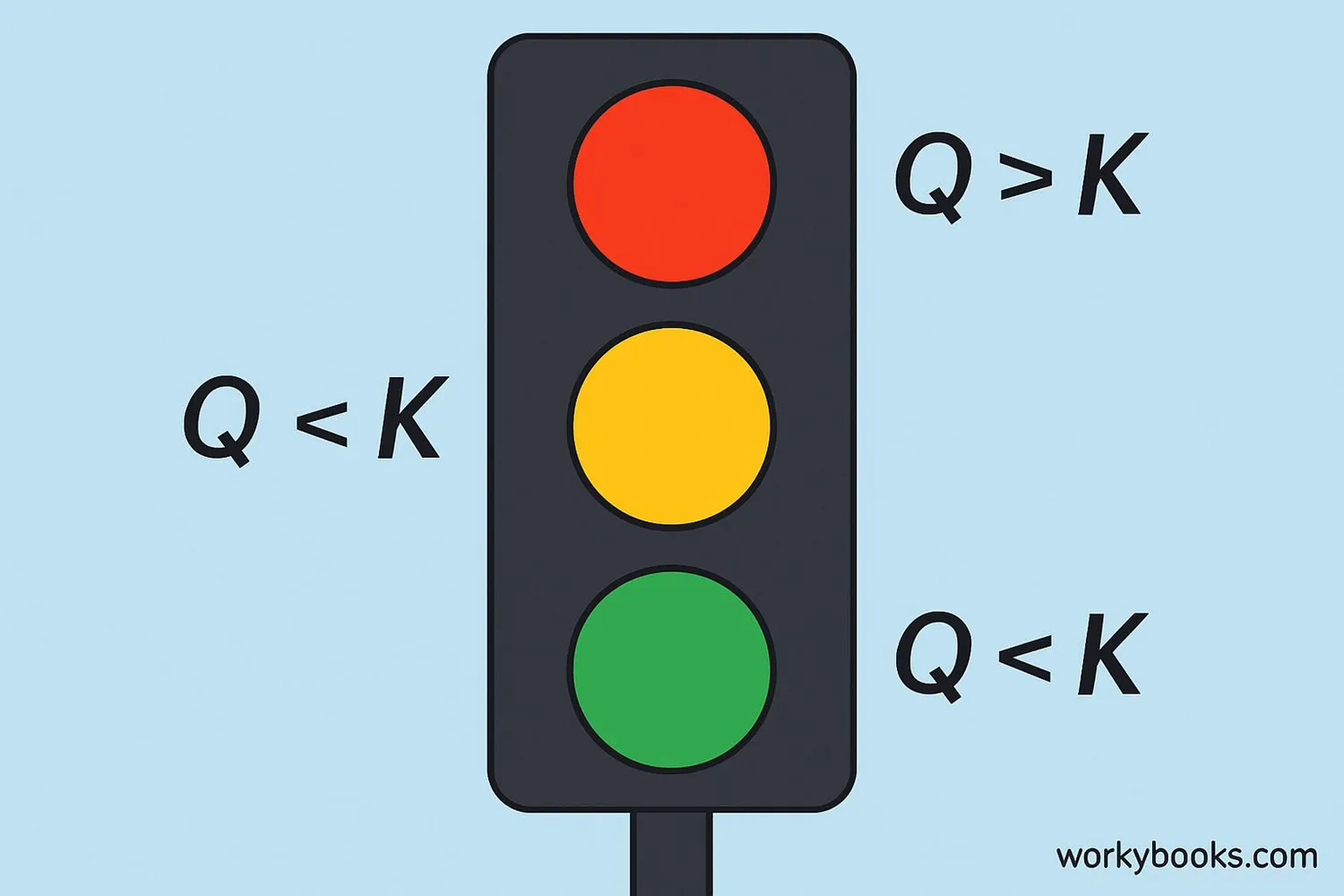
The reaction quotient (Q) is like K's younger sibling! It has the same formula as K, but we calculate it at any point during the reaction, not just at equilibrium. Q tells us which way the reaction needs to go to reach equilibrium.
Q < K
Reaction will move forward (make more products)
Q = K
Reaction is at equilibrium
Q > K
Reaction will move backward (make more reactants)
Think of Q as a GPS for chemical reactions! It tells the reaction: "You need to go this way to reach your destination (equilibrium)!" Scientists use Q to predict how reactions will behave before they reach balance.
Gibbs Free Energy
The Gibbs free energy (G) tells us if a reaction can happen spontaneously. When ΔG is negative, the reaction will move forward to reach equilibrium!
Equilibrium Quiz
Test your knowledge with this equilibrium quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about chemical equilibrium:
Fun Equilibrium Trivia
Discover some amazing facts about chemical equilibrium!
Ocean Balance
The ocean maintains a delicate equilibrium between CO₂ in the air and dissolved carbonate ions. This balance is crucial for marine life with shells!
Breathing Equilibrium
Your breathing is controlled by an equilibrium between oxygen and carbon dioxide in your blood. When CO₂ builds up, your brain tells you to breathe faster!
Universal Balance
Equilibrium concepts apply beyond chemistry! Stars maintain equilibrium between gravity pulling inward and nuclear reactions pushing outward.
Historical Discovery
The concept of chemical equilibrium was first clearly described by Norwegian chemists Cato Guldberg and Peter Waage in 1864. They called it the "Law of Mass Action".





