Lewis Dot Structures - Definition, Examples, Quiz, FAQ, Trivia
Discover how to represent atoms and molecules using electron dot diagrams!
What are Lewis Dot Structures?
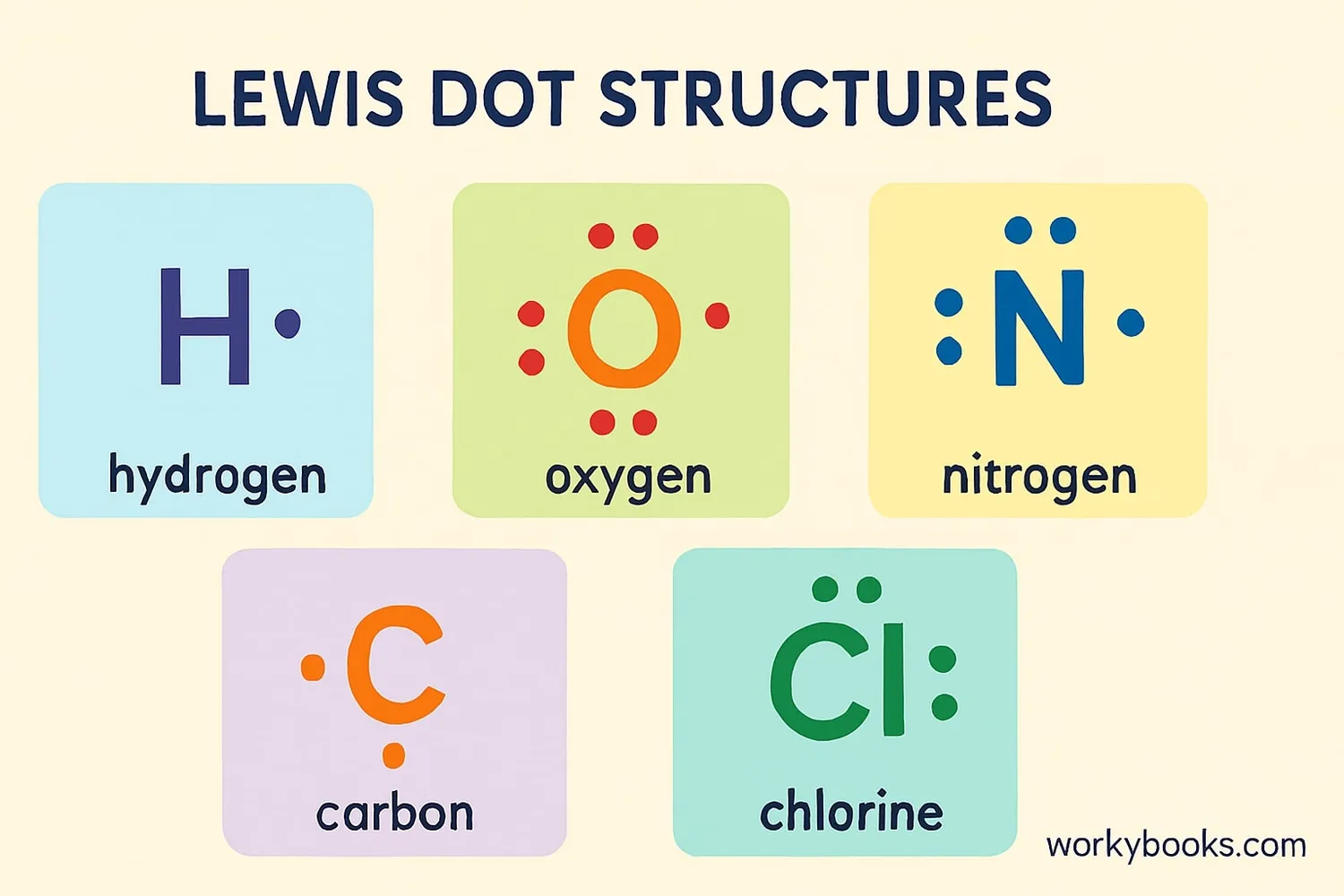
Lewis Dot Structures (also called electron dot diagrams) are simple drawings that show how atoms bond together to form molecules. They help us understand how atoms share or transfer electrons when they form chemical bonds.
These diagrams were invented by American chemist Gilbert Lewis in 1916. They use the chemical symbol of an element surrounded by dots that represent the valence electrons - the electrons in the outermost shell of an atom that participate in chemical bonding.
Key Concept: Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom. These are the electrons that participate in chemical bonding and determine how atoms will connect with each other.
How to Draw Lewis Dot Structures
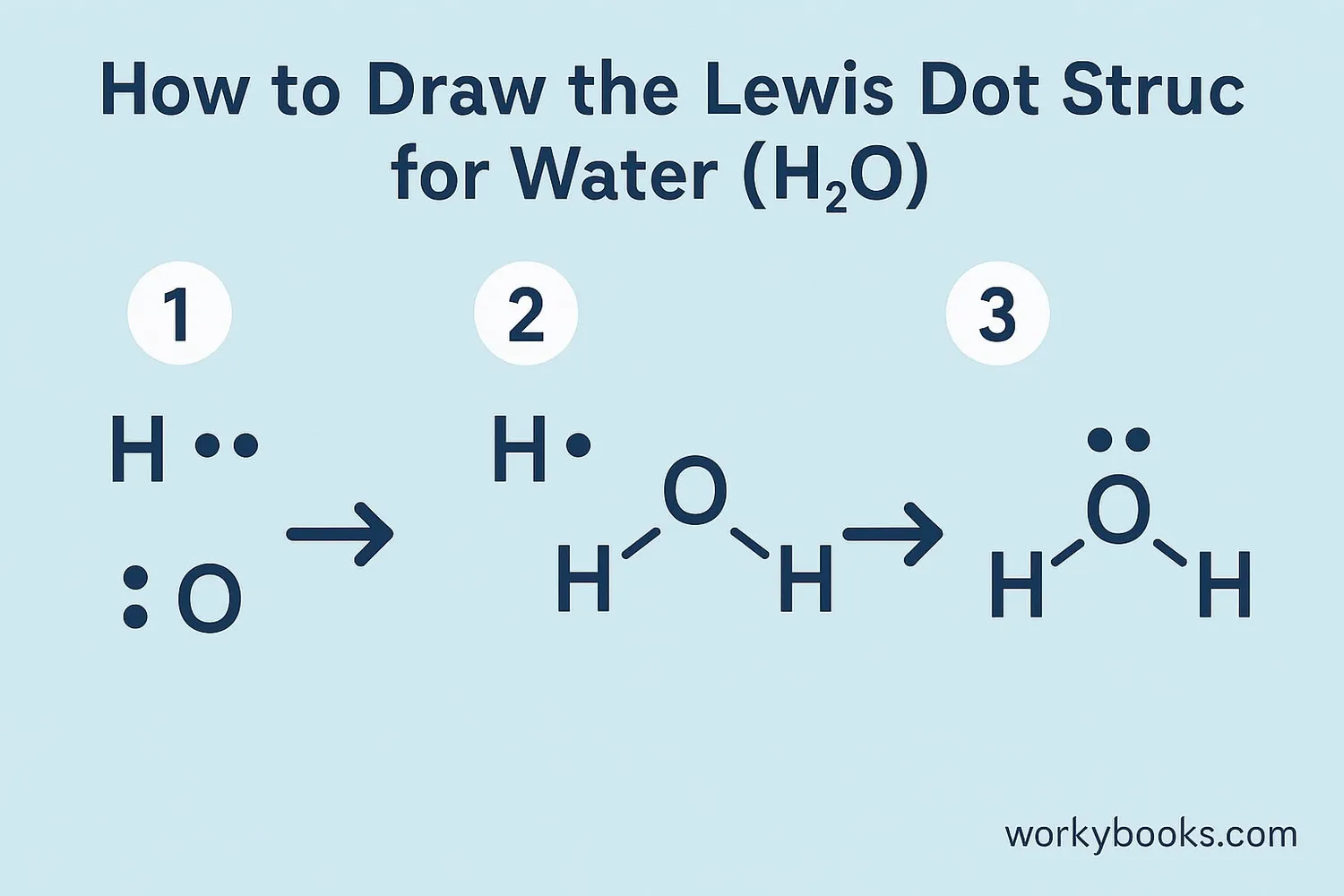
Drawing Lewis Dot Structures might seem tricky at first, but it becomes easier with practice. Here are the basic steps:
Find Valence Electrons
Determine how many valence electrons each atom has
Arrange Atoms
Place the least electronegative atom in the center
Connect Atoms
Use lines to show bonds between atoms
Add Lone Pairs
Place dots around atoms to show non-bonding electrons
Check Octet Rule
Ensure each atom has 8 electrons (except hydrogen)
Let's try an example with methane (CH₄):
1. Carbon has 4 valence electrons, hydrogen has 1 each
2. Carbon goes in the center
3. Draw lines connecting carbon to each hydrogen
4. Each bond uses 2 electrons (1 from carbon, 1 from hydrogen)
5. Carbon now has 8 electrons around it (4 bonds × 2 electrons each)
Remember the Octet Rule
Most atoms want to have 8 electrons in their outer shell to be stable. Hydrogen is the exception—it only needs 2 electrons to be stable.
Why Lewis Dot Structures Are Important
Lewis Dot Structures are more than just drawings—they help scientists understand and predict how molecules will behave. Here's why they're so useful:
Predict Bonding
Help predict how atoms will connect to form molecules
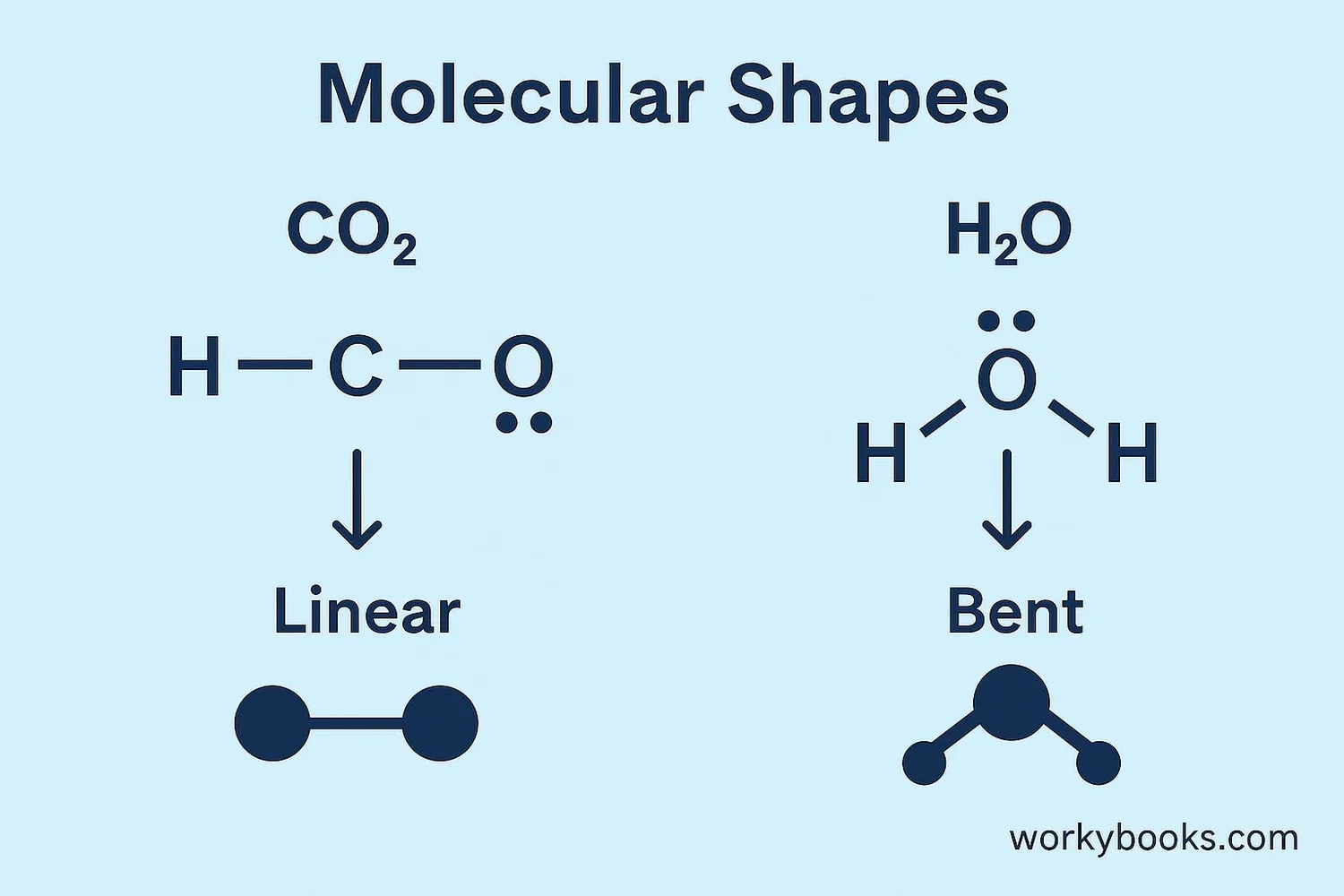
Determine Shape
Help determine the 3D shape of molecules
Understand Properties
Help explain why substances have certain properties
Lewis Dot Structures help us understand:
• Why water molecules are bent rather than straight
• How atoms share electrons in covalent bonds
• Why some molecules are polar and others are nonpolar
• How ionic compounds form when electrons are transferred
These diagrams are the foundation for understanding more complex concepts in chemistry!
Lewis Structures Knowledge Check
Test your understanding of Lewis Dot Structures with this quiz. Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about Lewis Dot Structures:
Interesting Facts About Lewis Structures
Discover some fascinating information about electron dot diagrams:
Historical Origins
Gilbert Lewis initially published his electron dot ideas in a 1916 article titled "The Atom and the Molecule." His ideas were revolutionary at the time and transformed how chemists understood chemical bonding.
Visual Learning
Research shows that students who learn using Lewis Dot Structures develop better spatial reasoning skills and understanding of molecular geometry compared to those who only learn through formulas.
Element Variations
While most elements follow predictable patterns in their Lewis Structures, transition metals often break the rules, which is why they're usually not represented with simple Lewis Dot diagrams.
Digital Applications
Modern chemistry software uses the principles of Lewis Structures to predict molecular shapes and properties, helping scientists design new materials and drugs without expensive laboratory experiments.





