Radioactivity - Definition, Examples, Quiz, FAQ, Trivia
Discover how some atoms release energy and what this means for our world
What is Radioactivity?
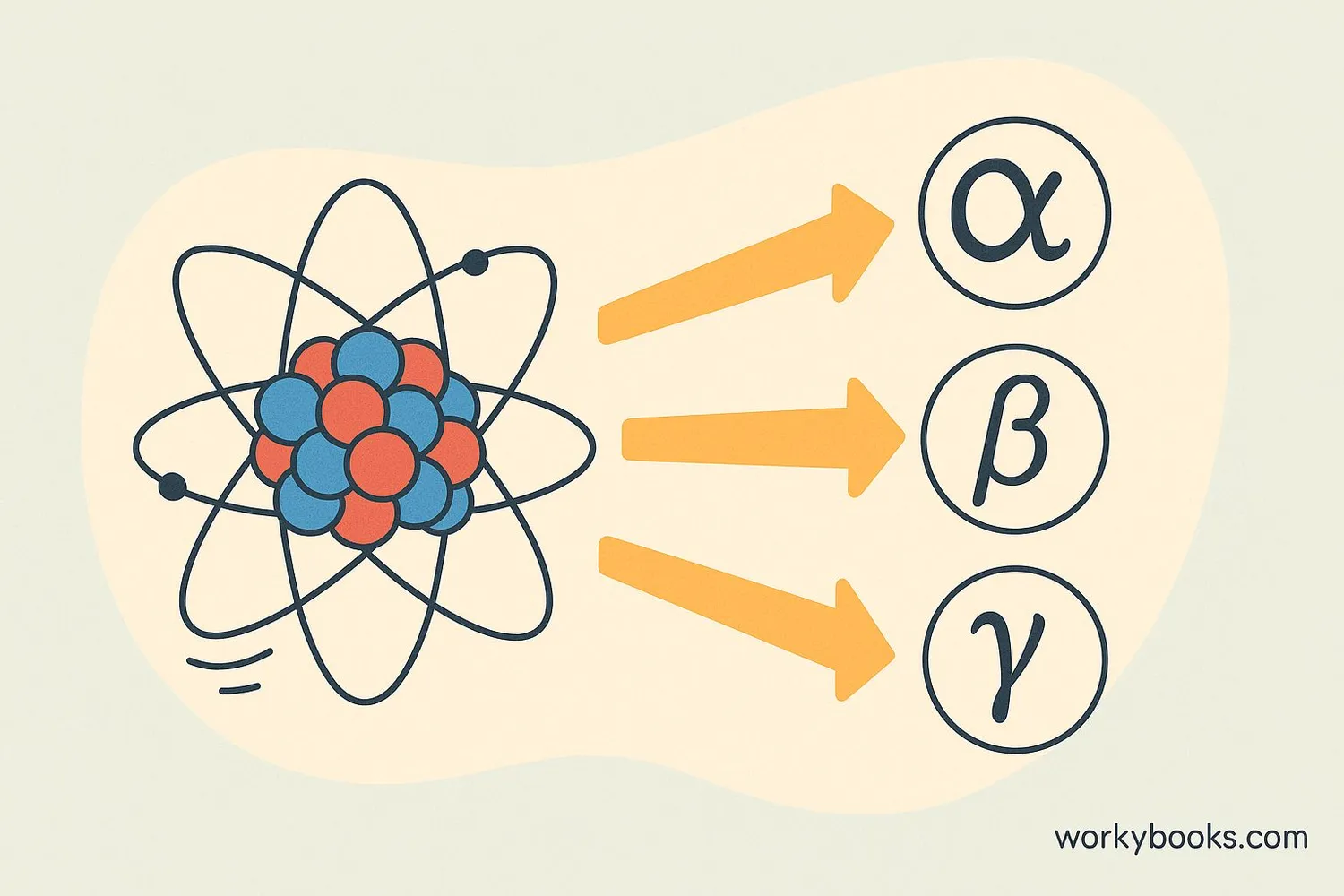
Radioactivity is a natural process where unstable atoms release energy to become more stable. This happens because some atoms have too much energy or mass to be stable. These unstable atoms are called radioactive isotopes.
Think of radioactive atoms like overfilled balloons. Just as balloons naturally let out air to become more stable, radioactive atoms release energy particles to become more balanced. This process is called radioactive decay or nuclear decay.
Science Fact!
Radioactivity was discovered by Henri Becquerel in 1896 when he noticed uranium salts could darken photographic plates without light!
How Radioactivity Works
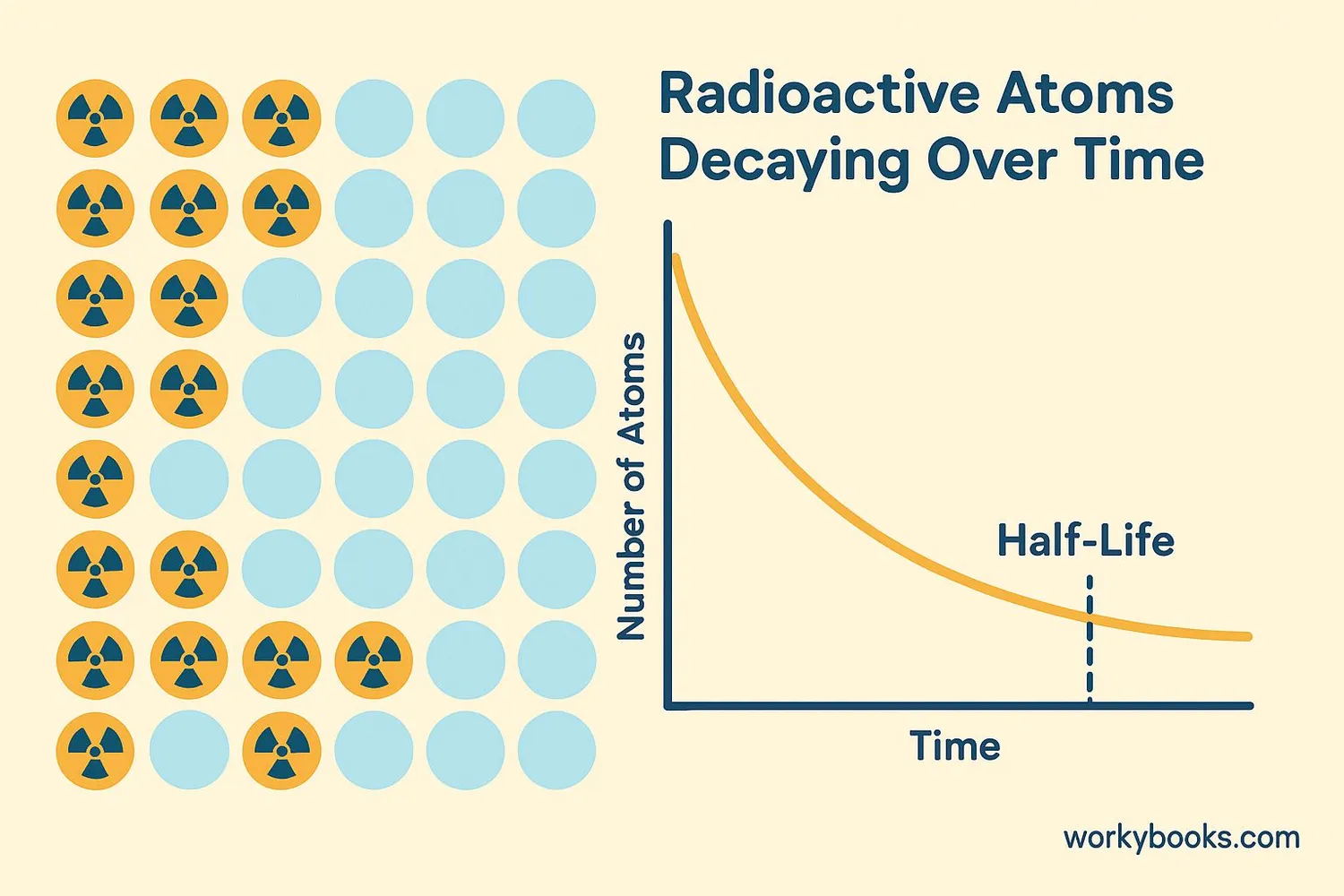
Radioactive decay happens when an unstable atomic nucleus releases energy to become more stable. This process occurs naturally and can't be sped up or slowed down by normal means. Scientists measure how quickly this happens using a concept called half-life - the time it takes for half of the radioactive material to decay.
Unstable Nucleus
Some atoms have an unstable combination of protons and neutrons
Energy Release
The atom releases particles or energy to become more stable
Transformation
The atom may change into a different element entirely
Stable State
After one or more decays, the atom reaches a stable form
Half-Life Example
Carbon-14 has a half-life of 5,730 years. This means if you start with 100 atoms of carbon-14, after 5,730 years you'll have 50 atoms left, and after 11,460 years you'll have 25 atoms left.
Types of Radiation
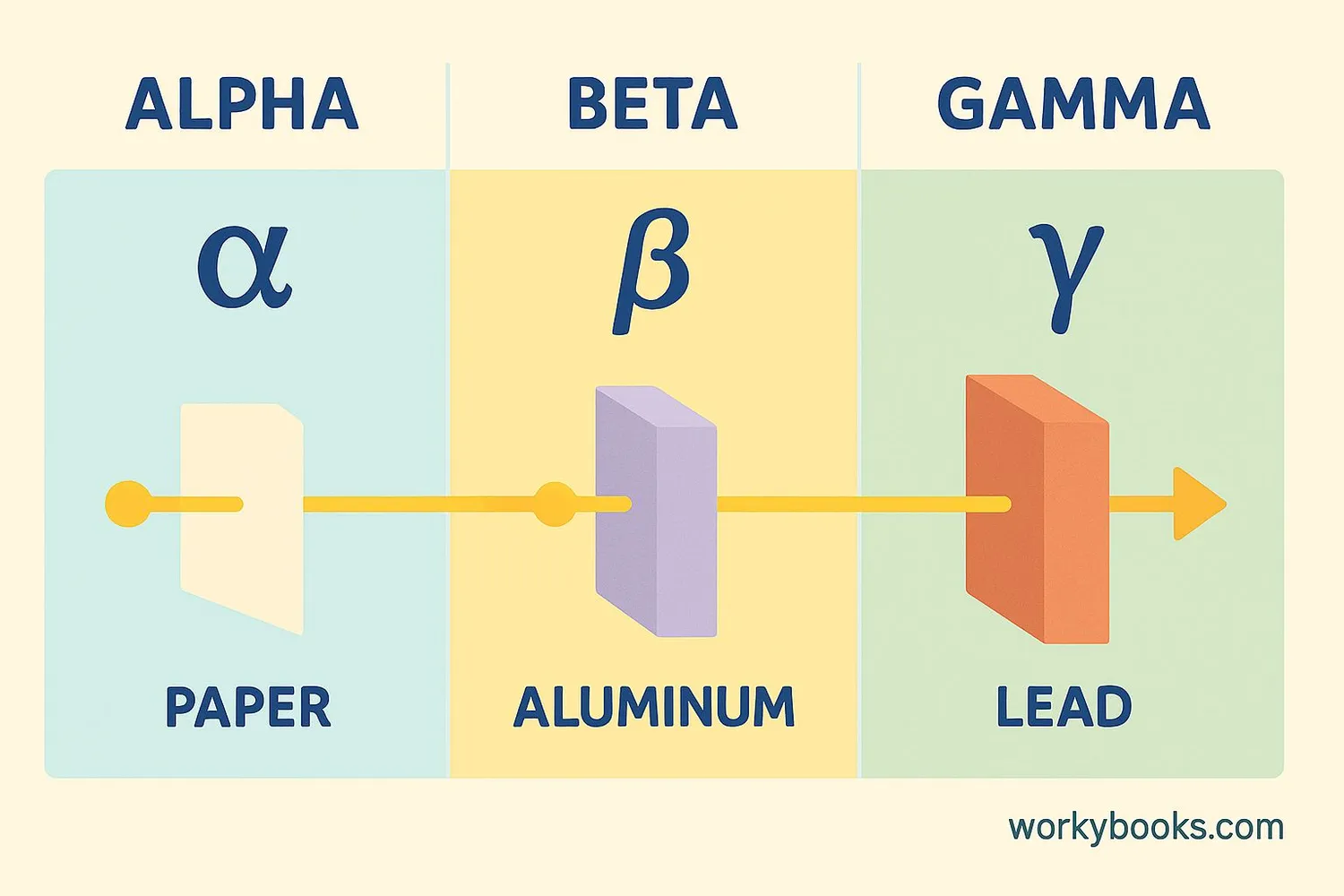
There are three main types of radiation emitted during radioactive decay. Each has different properties and requires different safety precautions:
Alpha Radiation
Made of 2 protons and 2 neutrons. Can be stopped by paper or skin but dangerous if swallowed or inhaled.
Beta Radiation
High-energy electrons. Can be stopped by aluminum foil or plastic but can penetrate skin.
Gamma Radiation
High-energy waves. Requires thick lead or concrete to block. Can penetrate deeply into materials and living tissue.
Scientists use special equipment like Geiger counters to detect and measure these different types of radiation. Understanding the differences helps us use radioactive materials safely and protect ourselves from unnecessary exposure.
Radiation Safety
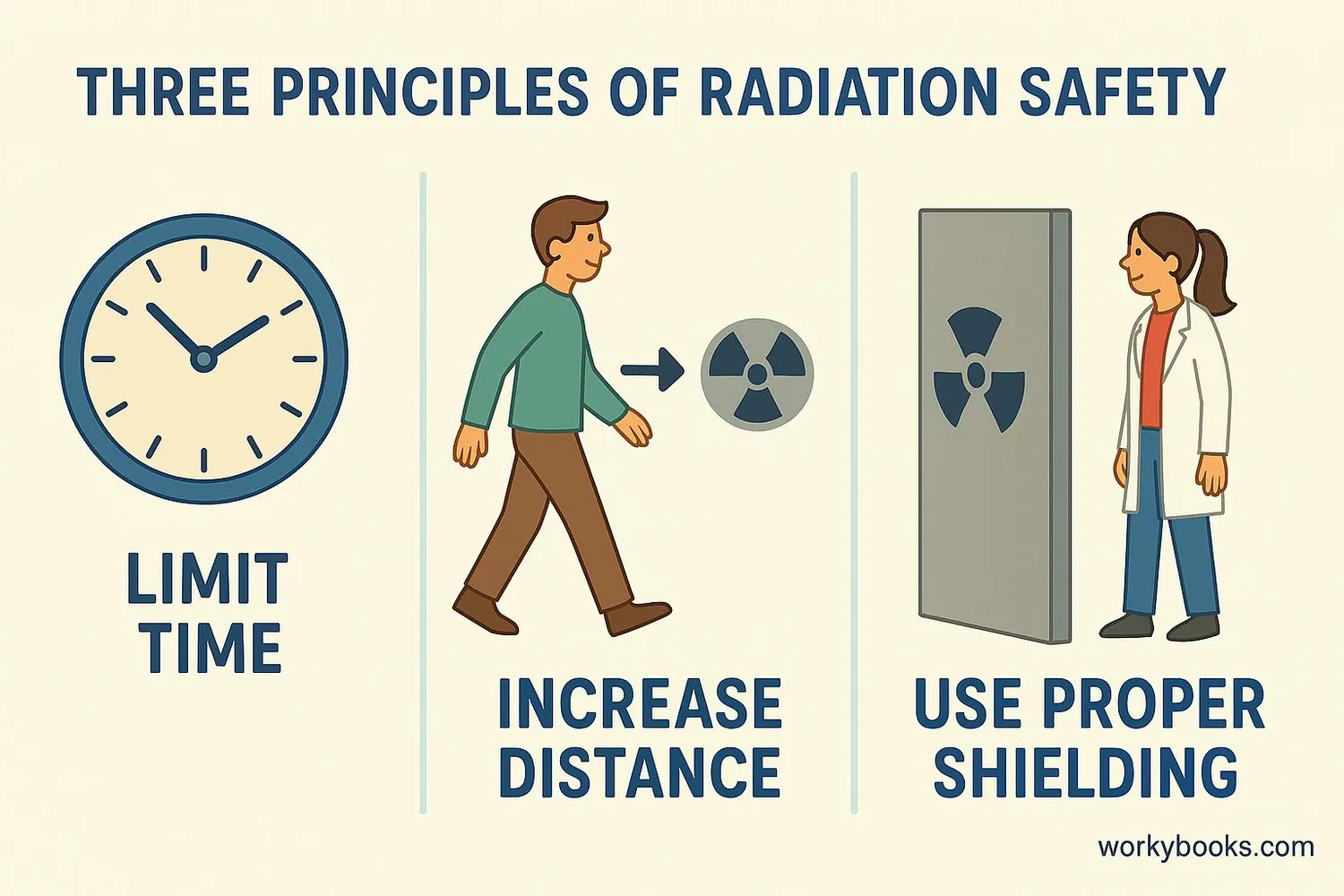
While radiation occurs naturally all around us, it's important to understand how to stay safe when around stronger sources. Scientists and doctors follow three important principles for radiation safety:
Time
Limit time spent near radiation sources. Less time means less exposure.
Distance
Stay as far away as possible from radiation sources. Radiation intensity decreases with distance.
Shielding
Use appropriate materials (lead, concrete) to block radiation between you and the source.
It's also important to understand that we're exposed to natural radiation every day from sources like:
• Rocks and soil (radon gas)
• Cosmic rays from space
• Even from within our own bodies (potassium-40)
This natural background radiation is typically at safe levels and doesn't pose health risks for most people.
Safety First!
Never handle unknown materials that might be radioactive. If you see a radiation warning symbol, keep your distance and tell an adult.
Radioactivity Knowledge Check
Test what you've learned about radioactivity with this quiz. Answer all 5 questions to see how much you understand.
Frequently Asked Questions
Here are answers to some common questions about radioactivity:
Science Facts About Radioactivity
Discover some fascinating facts about radioactivity:
Banana Equivalent Dose
Bananas contain potassium-40, a naturally radioactive isotope. Eating one banana exposes you to about 0.1 microsieverts of radiation - a unit sometimes called a "Banana Equivalent Dose."
Home Radiation
The largest source of natural radiation exposure for most people is radon gas, which seeps from the ground into buildings. Testing homes for radon is important for health safety.
Radioactive Dating
Scientists use radioactive isotopes like carbon-14 to determine the age of ancient artifacts and fossils. This process is called radiometric dating and has helped us understand Earth's history.
Space Exploration
NASA uses radioactive materials to power spacecraft that travel too far from the Sun to use solar power effectively. The Curiosity rover on Mars is powered by a radioisotope thermoelectric generator.





