Radioactivity - Definition, Examples, Quiz, FAQ, Trivia
Understanding nuclear decay and radiation in our world
What is Radioactivity?
Radioactivity is a natural process where unstable atoms release energy to become more stable. These atoms have too much energy or mass and need to release particles to reach a balanced state.
Think of it like a wobbly tower of blocks that eventually falls to become more stable. Radioactive atoms do something similar - they release energy and particles until they become stable.
This process happens naturally in certain elements like uranium, radium, and thorium. It was discovered by Henri Becquerel in 1896 when he noticed uranium salts could fog photographic plates without light.
Did You Know?
Radioactivity occurs naturally all around us! The Earth itself contains radioactive elements, and we're exposed to small amounts of radiation every day from rocks, soil, and even space.
Types of Radiation
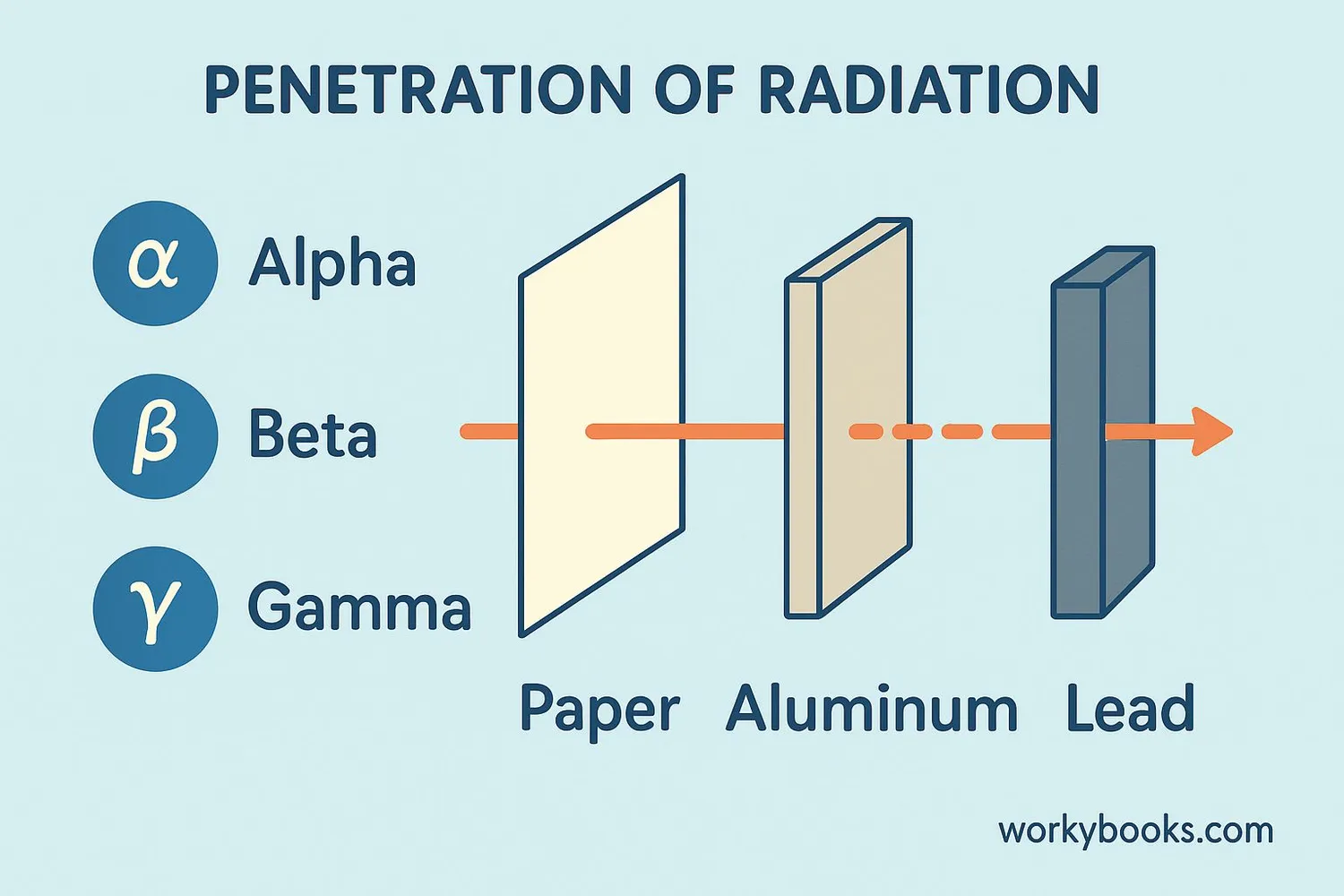
When radioactive atoms decay, they can release different types of particles and energy. The three main types are:
Alpha Particles
Made of 2 protons and 2 neutrons (like a helium nucleus). They're heavy, slow, and can be stopped by paper or skin.
Beta Particles
High-speed electrons ejected from the nucleus. They can penetrate further than alpha particles but can be stopped by aluminum.
Gamma Rays
High-energy electromagnetic waves (like X-rays but more powerful). They can penetrate deeply and require thick lead or concrete to stop.
Each type of radiation has different properties and uses. Alpha particles are used in smoke detectors, beta particles help measure material thickness, and gamma rays are used in medical treatments to kill cancer cells.
Radioactive Decay and Half-Life
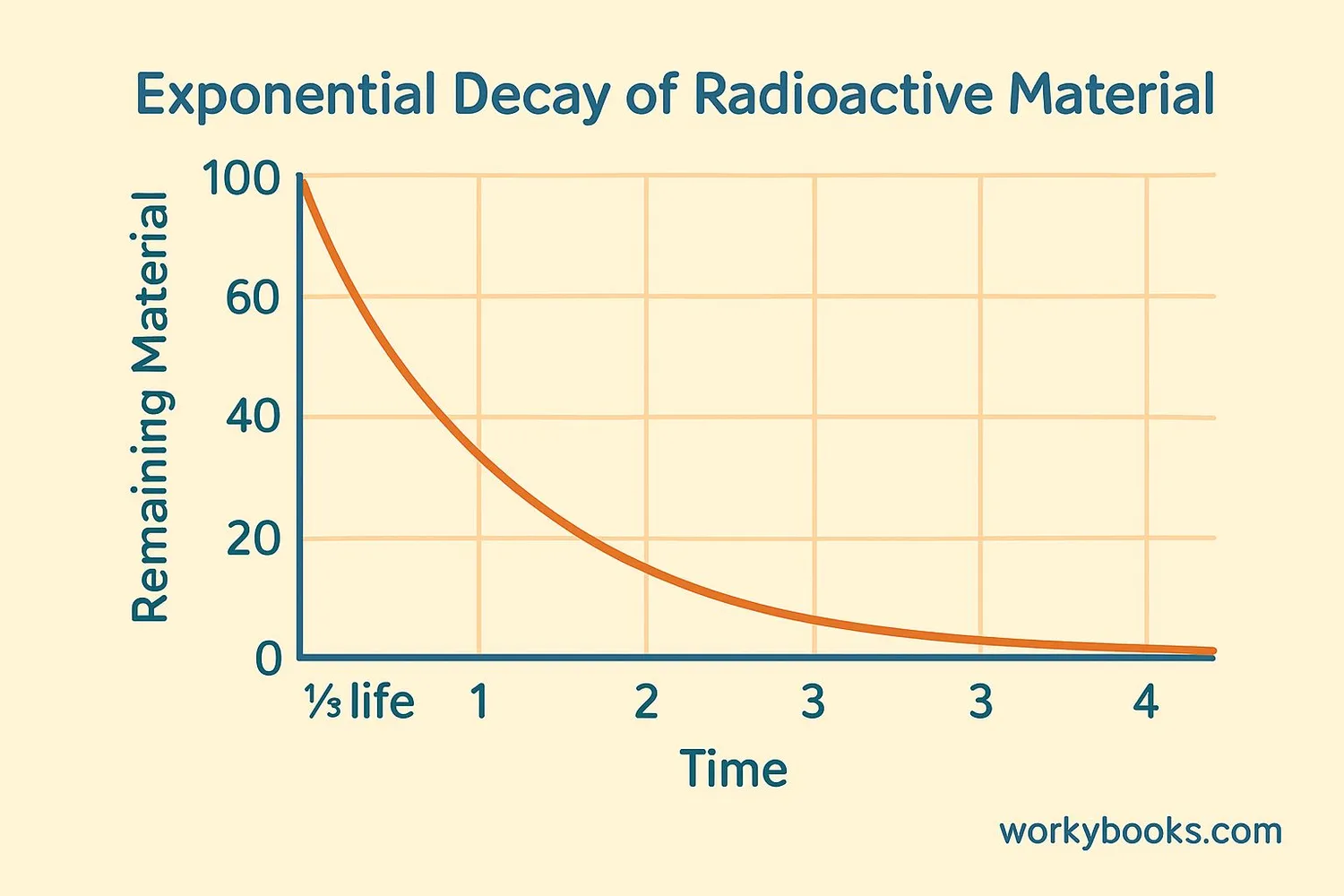
Radioactive decay happens at different rates for different elements. Scientists measure this using half-life - the time it takes for half of the radioactive atoms in a sample to decay.
For example, if we have 100 atoms of a radioactive element with a half-life of 1 year:
• After 1 year: 50 atoms remain
• After 2 years: 25 atoms remain
• After 3 years: 12-13 atoms remain
Different elements have vastly different half-lives. Carbon-14 has a half-life of about 5,730 years, which makes it useful for dating ancient objects. Uranium-238 has a half-life of about 4.5 billion years - almost the age of Earth!
Real-World Application
Scientists use half-life measurements to determine the age of fossils and archaeological artifacts through a process called radiometric dating.
Radiation Safety

While radiation has many beneficial uses, it's important to handle it safely. Scientists follow three key principles for radiation safety:
Time
Limit the time spent near radioactive sources to reduce exposure
Distance
Stay as far away from radioactive sources as possible - radiation intensity decreases with distance
Shielding
Use appropriate protective materials like lead, concrete, or water to block radiation
Radiation is measured in units called sieverts or rem. The average person receives about 3-6 millisieverts (mSv) of natural background radiation per year from sources like:
• Cosmic rays from space
• Radioactive elements in rocks and soil
• Natural radioactivity in our own bodies
• Medical procedures like X-rays
Workers with radioactive materials wear special badges called dosimeters that track their radiation exposure to ensure they stay within safe limits.
Radioactivity Knowledge Check
Test your understanding of radioactivity with this quiz. Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about radioactivity:
Interesting Radioactivity Facts
Discover some fascinating facts about radioactivity!
Banana Equivalent Dose
Bananas are naturally radioactive due to their potassium content! Eating one banana exposes you to about 0.1 microsieverts of radiation. This "Banana Equivalent Dose" is used to compare radiation levels.
Natural Nuclear Reactor
About 2 billion years ago, in what is now Gabon, Africa, natural conditions created the world's only known natural nuclear reactor where uranium deposits underwent sustained nuclear fission.
Life-Saving Radiation
Many smoke detectors use a tiny amount of americium-241, a radioactive material, to detect smoke. This technology has saved countless lives from fire dangers.
Home Radiation
Radon gas, a radioactive decay product of uranium in soil, is the second leading cause of lung cancer after smoking. It can accumulate in homes, which is why radon testing is important.





