Specific Heat Capacity - Definition, Examples, Quiz, FAQ, Trivia
Discover how different materials absorb heat at different rates
What is Specific Heat Capacity?
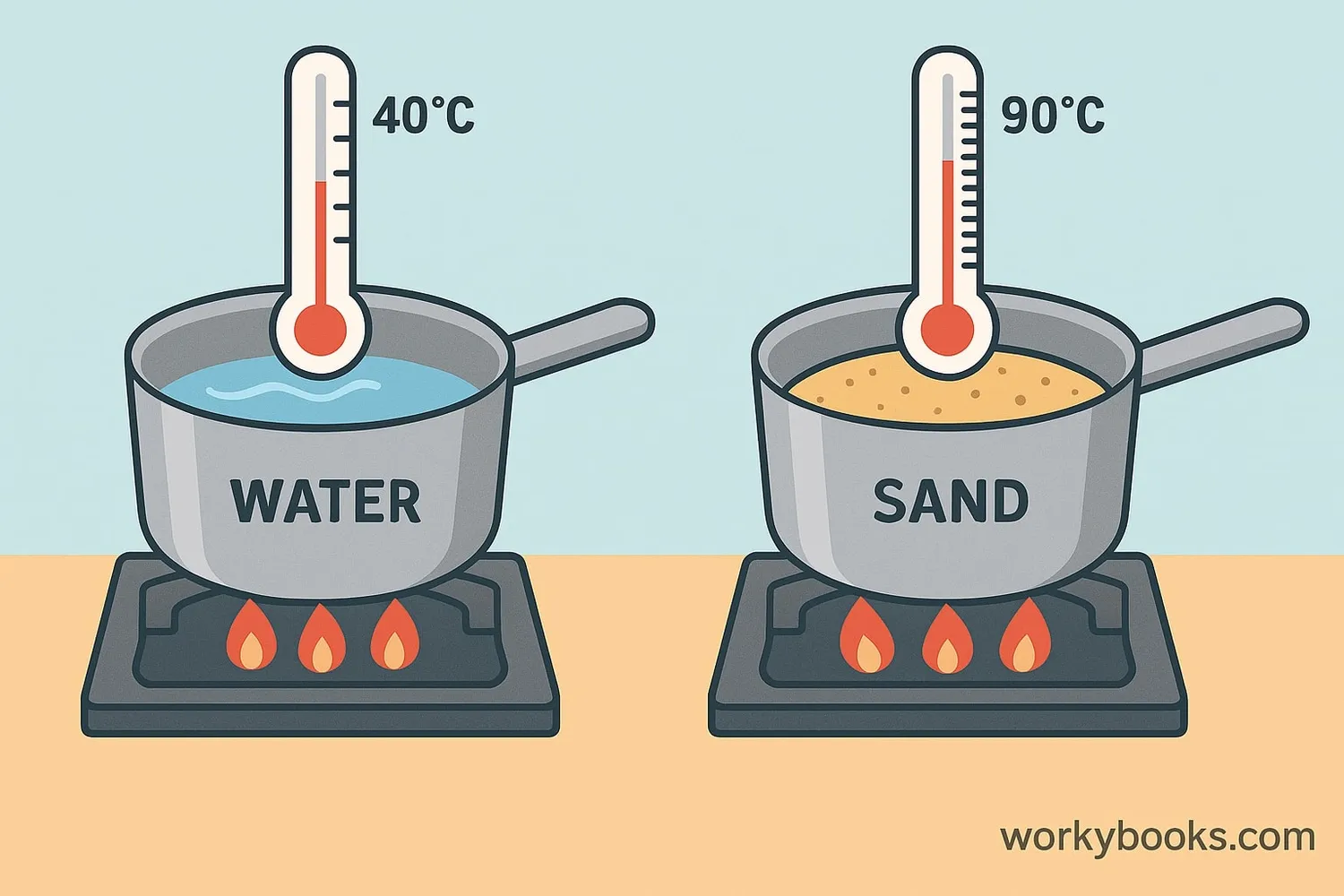
Specific heat capacity is a scientific concept that tells us how much heat energy a material can absorb before its temperature increases. Think of it as a measure of how "resistant" a material is to heating up.
Water has a very high specific heat capacity (4184 J/kg·K). This means it takes a lot of heat to raise water's temperature. Sand has a much lower specific heat capacity (about 800 J/kg·K), so it heats up quickly. This is why sand at the beach gets hot quickly on a sunny day, while water stays cooler.
The formula for specific heat capacity is:
Q = m × c × ΔT
Where:
• Q = heat energy (joules)
• m = mass (kilograms)
• c = specific heat capacity (J/kg·K)
• ΔT = change in temperature (Kelvin or Celsius)
Science Fact!
Water's high specific heat capacity helps regulate Earth's climate. Oceans absorb huge amounts of heat without changing temperature dramatically.
How Specific Heat is Measured

Scientists measure specific heat capacity using a device called a calorimeter. Here's how the process works:
Heat the Sample
A material sample is heated to a known temperature
Transfer to Water
The hot sample is placed in water at a cooler temperature
Measure Temperature
The temperature change of the water is measured
Calculate
The specific heat is calculated using energy conservation
Since energy is conserved, the heat lost by the sample equals the heat gained by the water. By measuring the temperature changes and masses, we can calculate the specific heat capacity using the formula:
m₁c₁ΔT₁ = m₂c₂ΔT₂
Where:
• m₁, c₁ = mass and specific heat of sample
• m₂, c₂ = mass and specific heat of water
• ΔT = temperature change
Measurement Units
Specific heat capacity is measured in joules per kilogram per kelvin (J/kg·K). Water's specific heat is 4184 J/kg·K - one of the highest of common materials!
Specific Heat of Common Materials
Different materials have very different specific heat capacities. This table shows how much heat energy is needed to raise 1kg of each material by 1°C:
| Material | Specific Heat (J/kg·K) | Heating Behavior |
|---|---|---|
| Water | 4184 | Heats and cools very slowly |
| Ice | 2093 | Heats about twice as fast as water |
| Steam | 2010 | Similar to ice |
| Aluminum | 897 | Heats about 4.5 times faster than water |
| Wood | 1700 | Heats about 2.5 times faster than water |
| Iron | 449 | Heats about 9 times faster than water |
| Copper | 385 | Heats about 11 times faster than water |
Metal Behavior
Metals like copper and iron have low specific heat capacities, which is why metal objects feel hot quickly when left in the sun.
Applications of Specific Heat
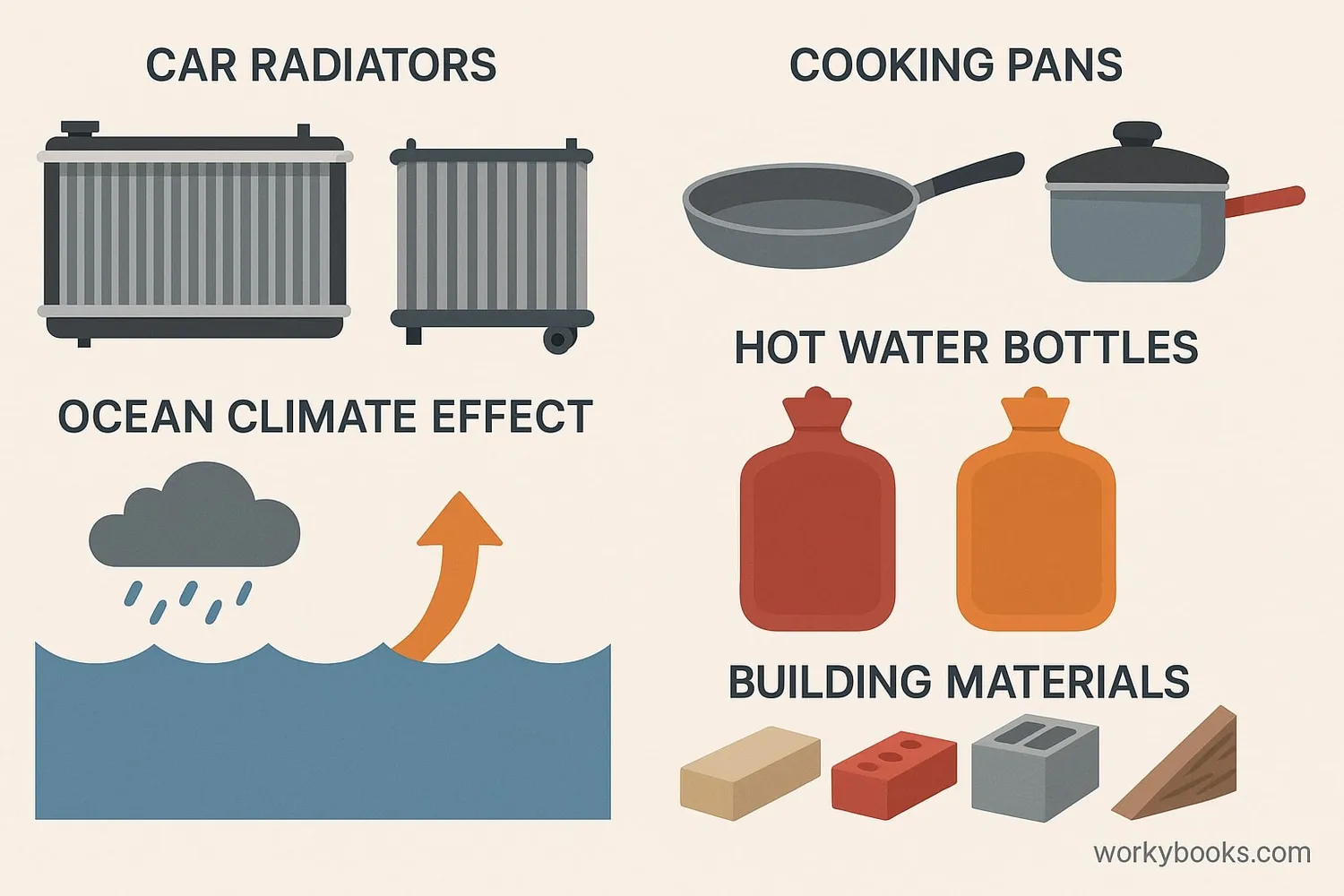
Specific heat capacity affects many aspects of our daily lives and technology:
Car Radiators
Water's high specific heat makes it excellent for absorbing engine heat
Cooking
Copper pans heat quickly and evenly due to low specific heat
Building Materials
Materials with high specific heat help regulate indoor temperatures
Other important applications include:
• Climate systems: Oceans moderate coastal temperatures
• Heating systems: Hot water heating systems are efficient
• Thermal storage: Materials like water store solar energy
• Safety equipment: Firefighters' suits use materials with high specific heat
Understanding specific heat helps engineers design better systems for cooking, transportation, and energy management.
Specific Heat Quiz
Test your knowledge with this interactive quiz on specific heat capacity:
Frequently Asked Questions
Here are answers to common questions about specific heat capacity:
Specific Heat Trivia
Discover amazing facts about specific heat capacity:
Water's Superpower
Water has one of the highest specific heat capacities of any common liquid. This unique property makes Earth habitable by preventing extreme temperature fluctuations.
Human Body Advantage
The human body is 60% water, which gives us thermal stability. Our high water content helps maintain a constant body temperature despite environmental changes.
Record Holder
Hydrogen gas has the highest specific heat capacity of all elements at 14,300 J/kg·K. This is why hydrogen is used as a coolant in large generators.
Solar Power Solution
Solar power plants often use molten salt for thermal storage because it has a high specific heat capacity (about 1500 J/kg·K), allowing it to store large amounts of heat energy.





