Thermal Equilibrium - Definition, Examples, Quiz, FAQ, Trivia
Understanding How Heat Flows and Temperatures Balance
What is Thermal Equilibrium?
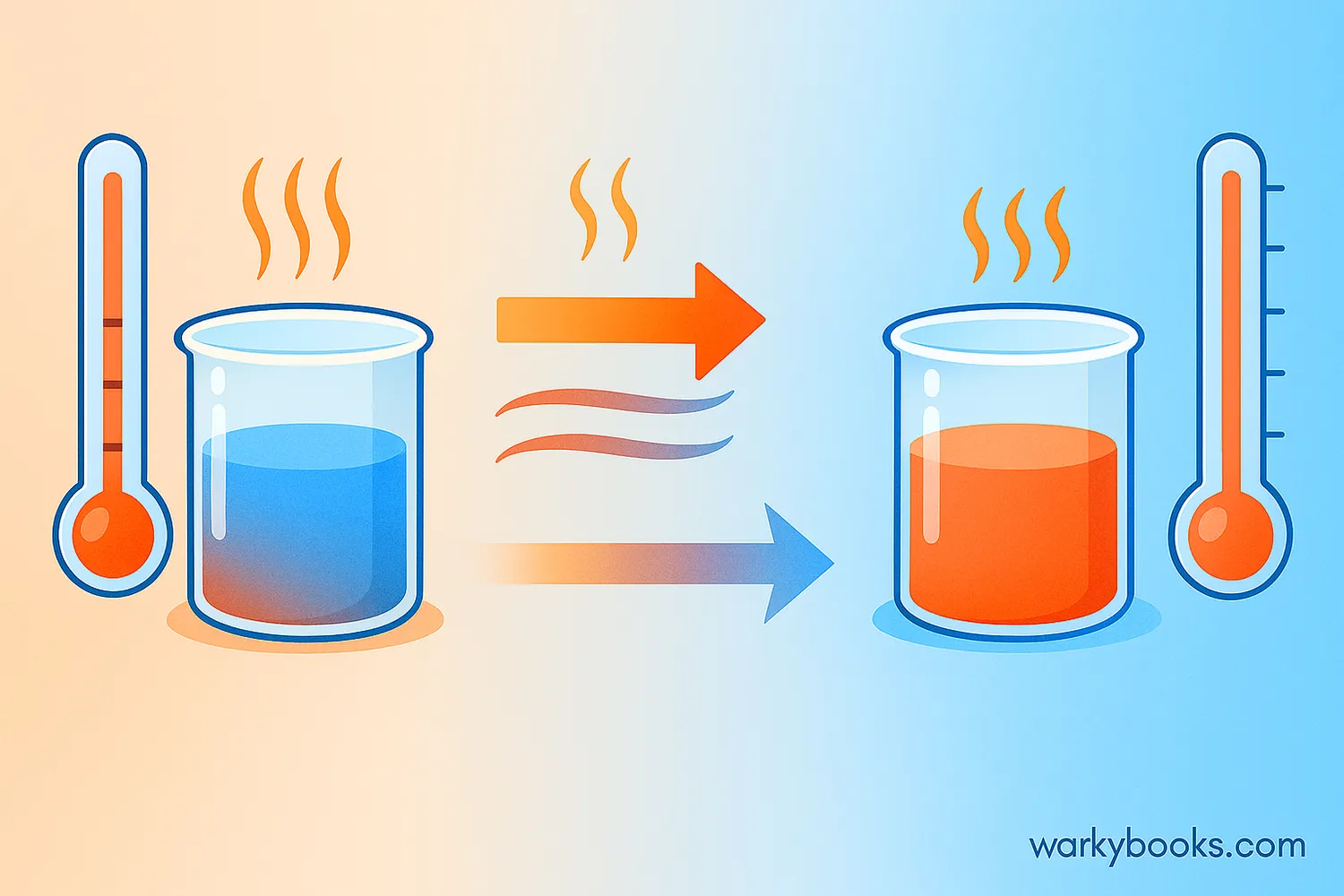
Thermal equilibrium is a state where two objects at different temperatures eventually reach the same temperature when they're in contact with each other. When this happens, there's no more net flow of heat between them.
Think about what happens when you add ice to a warm drink. At first, the ice is cold and the drink is warm. But after some time, they both reach the same temperature - your drink becomes cooler, and the ice melts. When they're the same temperature, they've reached thermal equilibrium!
Science Fact!
The concept of thermal equilibrium is fundamental to understanding how heat works. It helps explain why objects feel hot or cold to the touch and how temperatures balance out in nature.
Zeroth Law of Thermodynamics
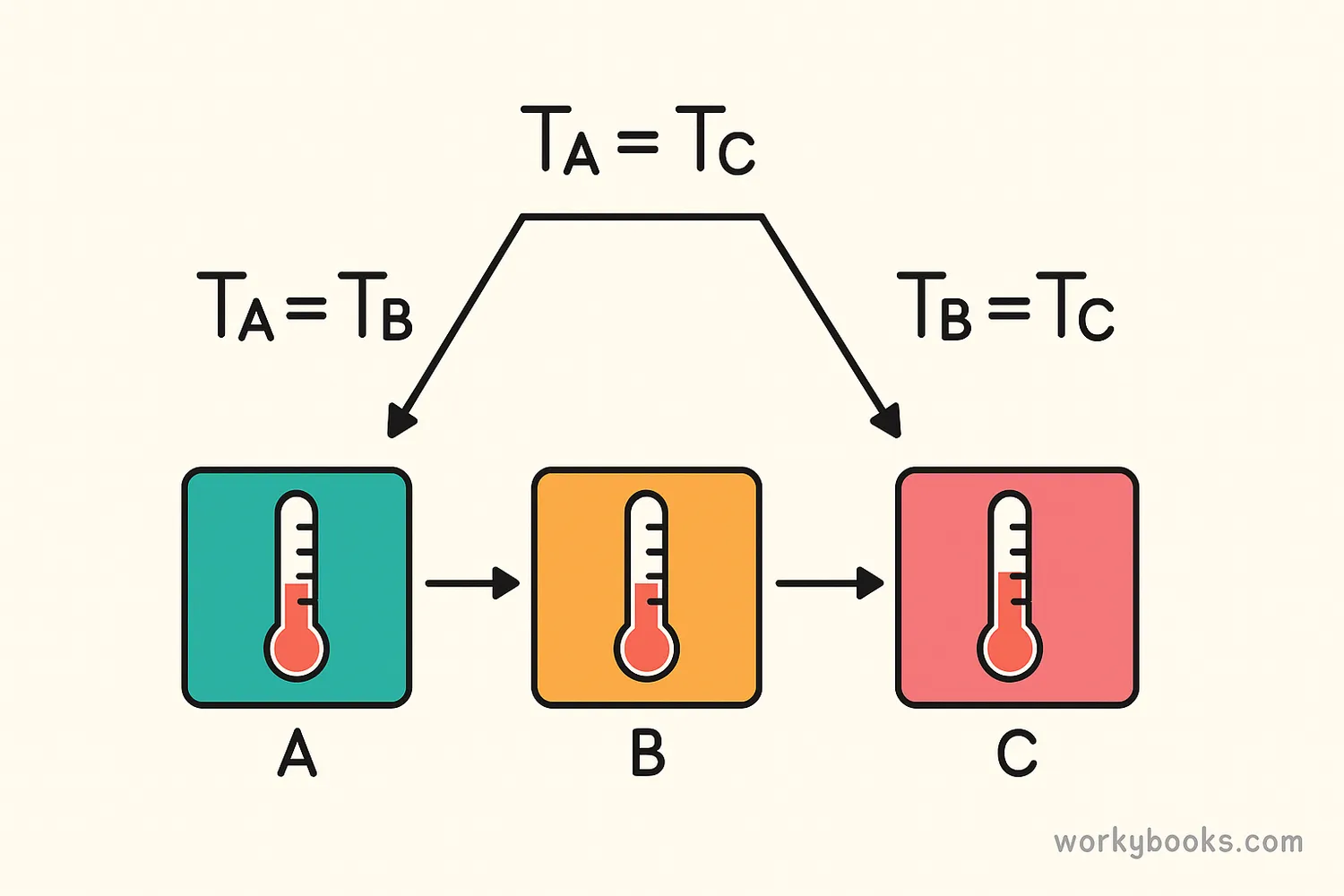
The Zeroth Law of Thermodynamics is all about thermal equilibrium. It says that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
This might sound complicated, but it's actually simple:
If A = B
If object A is the same temperature as object B
And B = C
And object B is the same temperature as object C
Then A = C
Then object A must be the same temperature as object C
This law might seem obvious to us now, but it's actually the foundation for how thermometers work! It allows us to compare temperatures of different objects accurately.
(in terms of temperature)
Heat Transfer
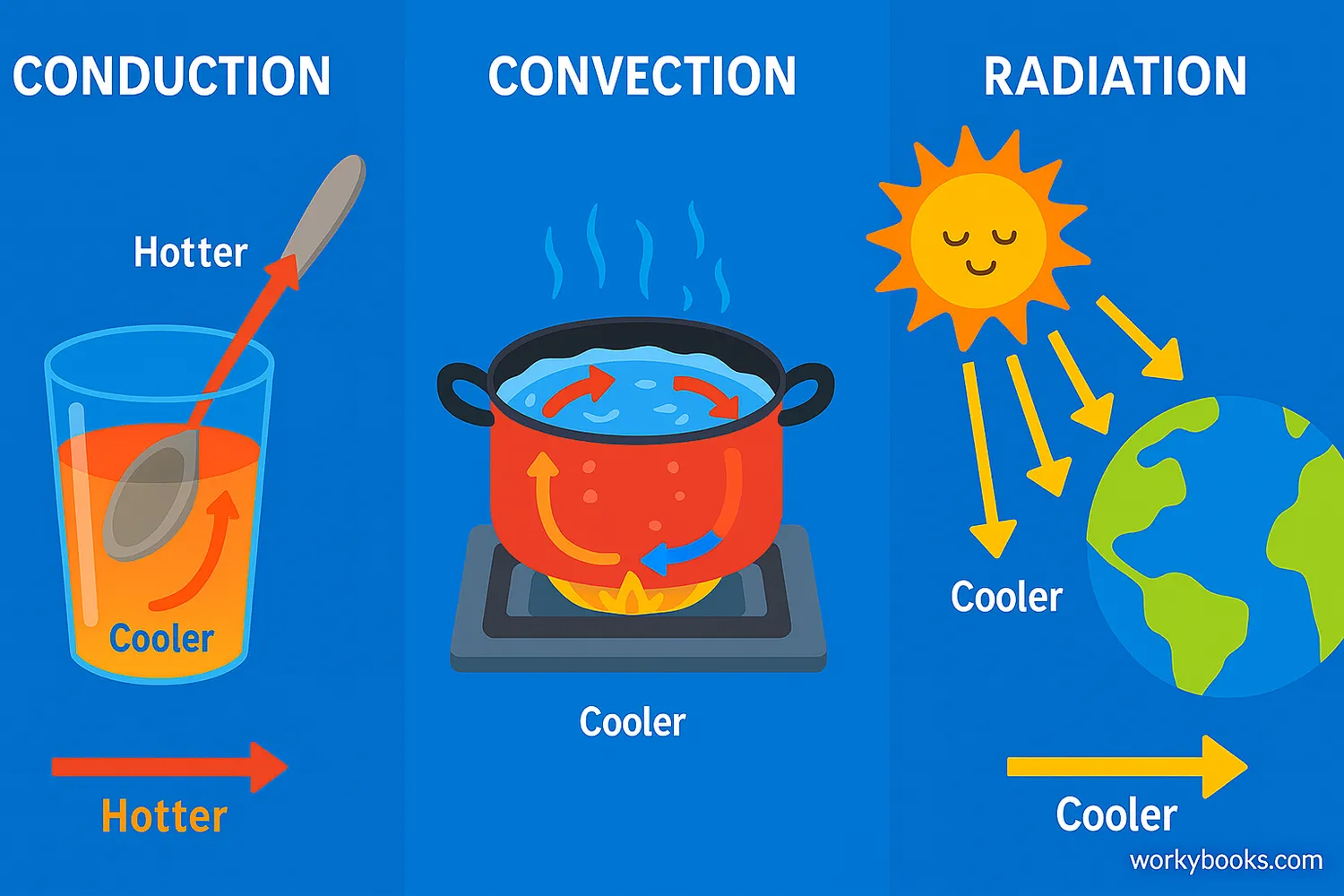
Heat always flows from warmer objects to cooler objects until thermal equilibrium is reached. This happens through three main processes:
| Method | How It Works | Example |
|---|---|---|
| Conduction | Direct contact between objects | A spoon getting hot in soup |
| Convection | Movement of fluids (liquids or gases) | Hot air rising, cool air sinking |
| Radiation | Transfer through electromagnetic waves | Sun warming the Earth |
No matter which method transfers the heat, the result is the same: the warmer object cools down, the cooler object warms up, and eventually they reach the same temperature.
Specific Heat Capacity
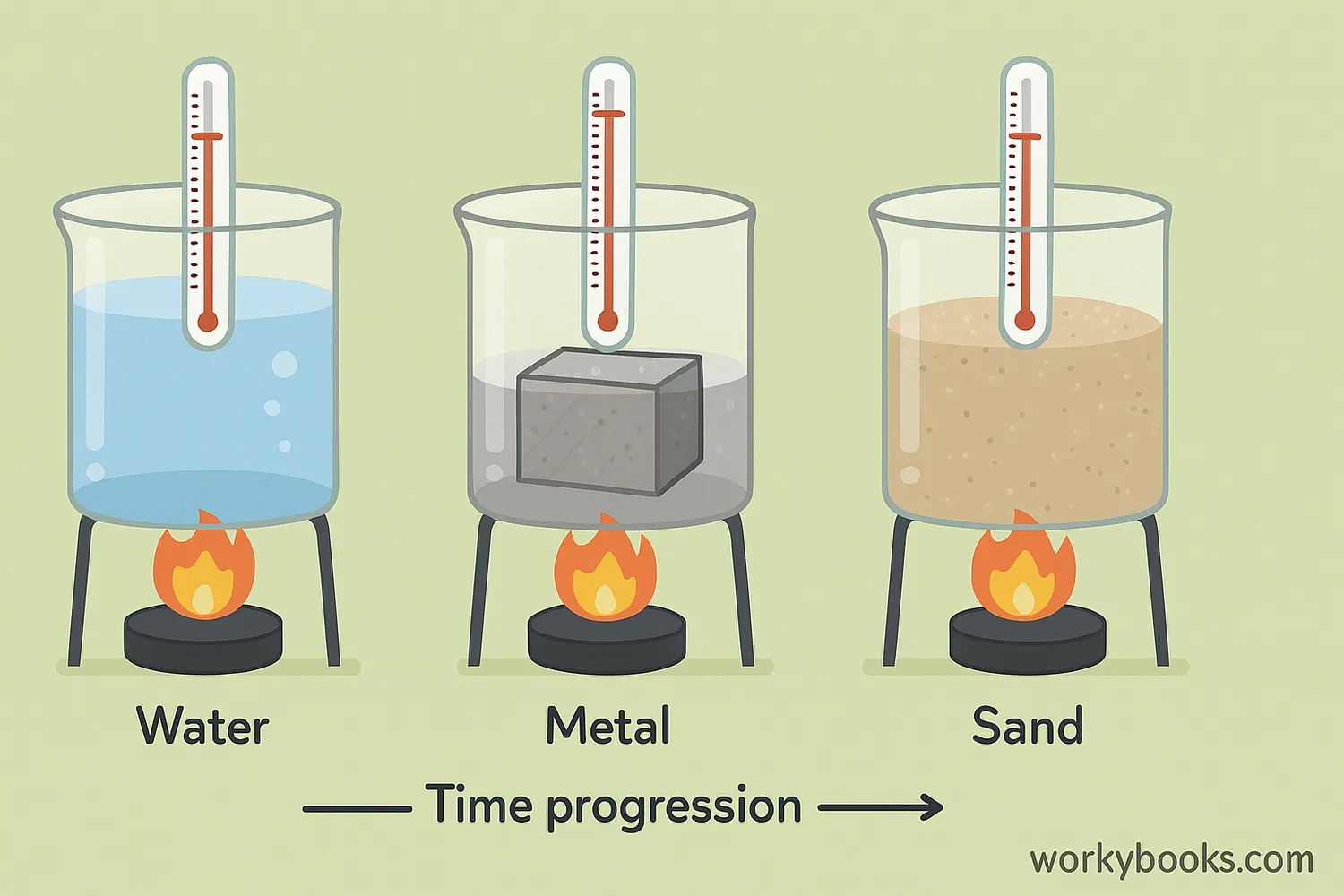
Not all substances heat up or cool down at the same rate! This is because of a property called specific heat capacity - the amount of heat energy needed to raise the temperature of 1 gram of a substance by 1°C.
Substances with high specific heat capacity (like water) need more energy to change temperature, while substances with low specific heat capacity (like metals) change temperature quickly.
Water
High specific heat (4.184 J/g°C)
Heats and cools slowly
Metals
Low specific heat (~0.4 J/g°C)
Heat and cool quickly
Sand
Medium specific heat (~0.8 J/g°C)
Moderate heating/cooling rate
This is why on a sunny day, sand at the beach gets hot quickly, while water stays cool. It's also why coastal areas have milder temperatures than inland areas - the ocean's high specific heat capacity helps regulate temperature.
Examples of Thermal Equilibrium
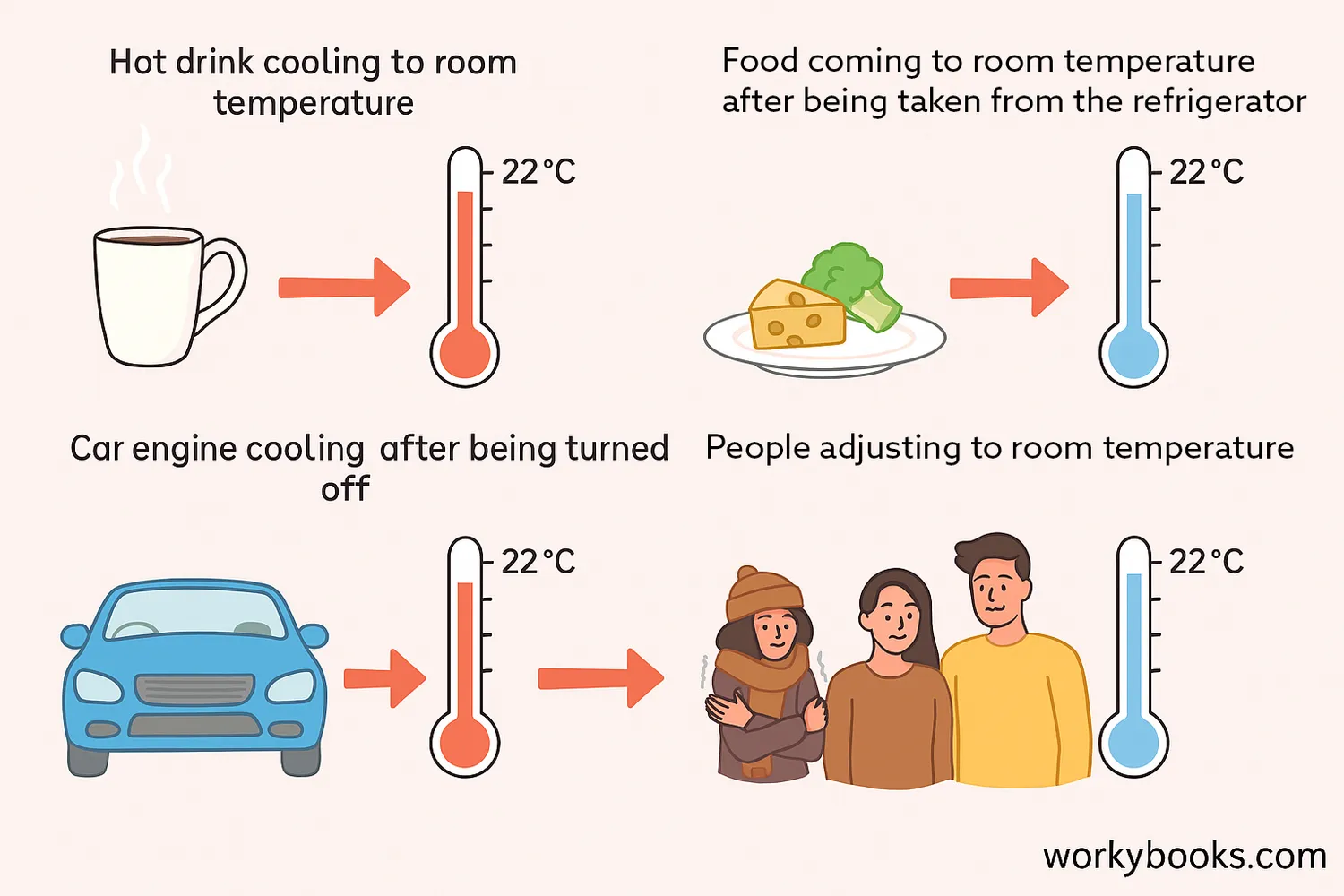
Thermal equilibrium is happening all around us every day. Here are some common examples:
| Example | What Happens | Time to Reach Equilibrium |
|---|---|---|
| Hot drink cooling | Heat flows from drink to air until same temperature | 10-30 minutes |
| Food from refrigerator | Food warms up to room temperature | 30-60 minutes |
| Car engine cooling | Engine cools to ambient temperature after use | Several hours |
| Human body adjustment | Body adjusts to room temperature (through sweating or shivering) | Few minutes |
Our bodies are constantly working to maintain thermal equilibrium with our environment. When we're hot, we sweat to cool down. When we're cold, we shiver to warm up. This helps keep our internal temperature stable at around 37°C (98.6°F).
Thermal Equilibrium Quiz
Test your knowledge with this quiz! Answer all 5 questions to see how much you've learned about thermal equilibrium.
Frequently Asked Questions
Here are answers to some common questions about thermal equilibrium:
Science Facts About Thermal Equilibrium
Discover some fascinating facts about thermal equilibrium and temperature!
The Universe's Temperature
The average temperature of the universe is just 2.7 Kelvin (-270.45°C or -454.81°F)! This is the temperature of the cosmic microwave background radiation left over from the Big Bang.
Animal Temperature Regulation
Many animals use behavioral thermoregulation to maintain thermal equilibrium with their environment. Lizards bask in the sun to warm up and seek shade to cool down, effectively managing their body temperature.
Spacecraft Temperature Control
Spacecraft must manage extreme temperature differences in space. The side facing the sun can reach 120°C (248°F), while the shaded side can drop to -100°C (-148°F). Spacecraft use special coatings and rotating systems to maintain thermal equilibrium.
Ocean's Thermal Mass
The ocean has an enormous thermal capacity due to water's high specific heat. It absorbs heat during the day and releases it at night, helping to regulate Earth's temperature and create milder coastal climates.





