Uranium - Definition, Examples, Quiz, FAQ, Trivia
Discover how this special element creates nuclear energy and powers our world!
What is Uranium?
Uranium is a special type of metal found in rocks all around the world. It's what scientists call a radioactive element, which means its atoms are always slowly breaking apart and releasing energy. This process is called radioactive decay.
Uranium has an atomic number of 92, which means each uranium atom has 92 protons in its center (called the nucleus). This makes it one of the heaviest elements found in nature. It was discovered in 1789 by a German chemist named Martin Klaproth.
Element Fact!
Uranium is about 40 times more common than silver in Earth's crust!
Properties of Uranium
Uranium has some special properties that make it different from other elements:
Radioactivity
Uranium atoms naturally break down over time, releasing energy
Density
It's very heavy - about 70% denser than lead
Isotopes
Uranium has different forms called isotopes (U-235 and U-238)
Reactivity
It reacts with air and tarnishes (gets a coating)
The most important isotopes (different forms) of uranium are U-235 and U-238. U-235 is special because it can easily undergo nuclear fission - a process where atoms split apart and release a huge amount of energy. This makes it perfect for use in nuclear reactors.
Isotope Ratio!
Only about 0.7% of natural uranium is the U-235 type needed for nuclear power. The rest is mostly U-238.
How Uranium Is Used
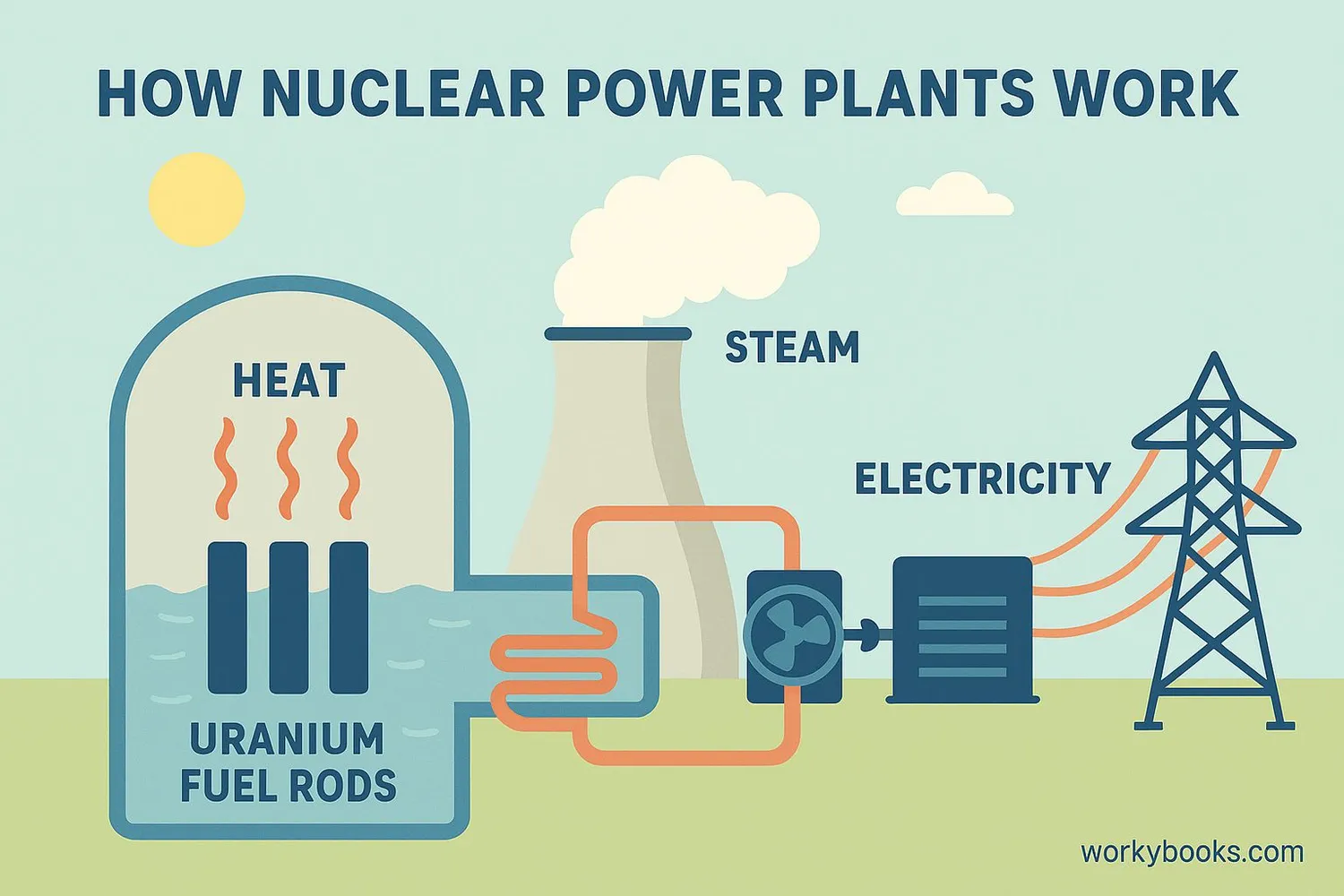
Uranium's main use is as a nuclear fuel to generate electricity. Here's how it works in a nuclear reactor:
Nuclear Fission
U-235 atoms split, releasing heat energy and neutrons
Heat Production
The splitting atoms create tremendous heat
Steam Generation
Heat turns water into steam that spins turbines
Electricity
Turbines power generators that create electricity
Nuclear power plants provide about 10% of the world's electricity. Unlike fossil fuels, nuclear power doesn't produce air pollution or greenhouse gases. A tiny amount of uranium can produce a huge amount of energy - one uranium fuel pellet (about the size of a pencil eraser) contains as much energy as 1 ton of coal!
Uranium Mining and Processing
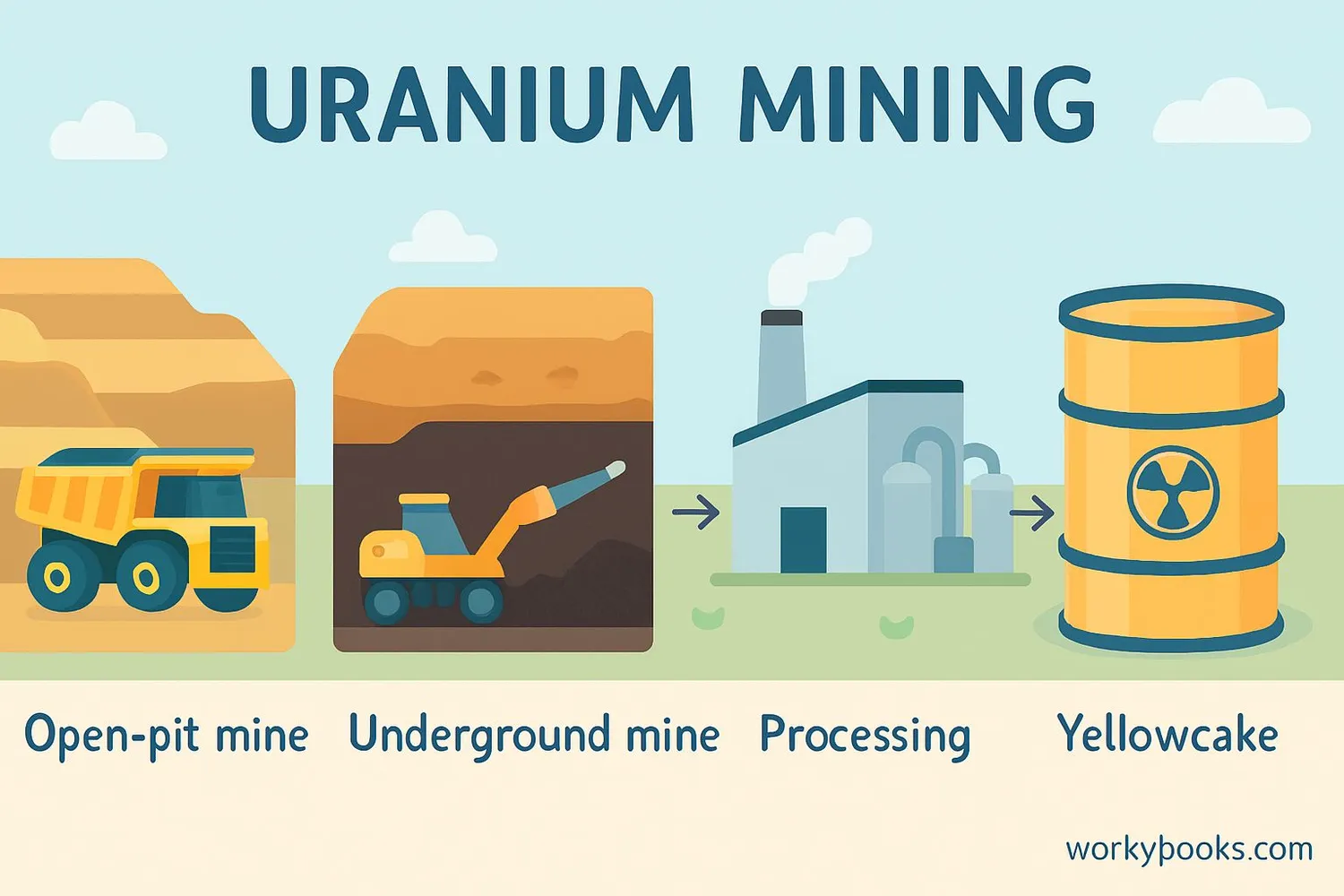
Uranium doesn't come out of the ground ready to use in nuclear reactors. It goes through several important steps:
Mining
Uranium ore is extracted from underground or open-pit mines
Milling
Ore is crushed and treated with chemicals to extract uranium
Conversion
Uranium is converted to uranium hexafluoride gas
Enrichment
U-235 concentration is increased through enrichment
Fuel Production
Enriched uranium is made into ceramic fuel pellets
Uranium enrichment is the process of increasing the percentage of U-235 in uranium. Natural uranium only contains about 0.7% U-235, but most nuclear power plants need fuel that is 3-5% U-235. This process requires special equipment and careful handling because uranium is a radioactive material.
Yellowcake!
After milling, uranium is in a form called "yellowcake" which is then processed further for use in reactors.
Uranium Knowledge Check
Test what you've learned about uranium with this quiz. Answer all 5 questions to check your understanding.
Frequently Asked Questions
Here are answers to some common questions about uranium:
Interesting Uranium Facts
Discover some amazing facts about uranium!
Ancient Natural Reactor
About 2 billion years ago, in what is now Gabon, Africa, natural conditions created a nuclear fission reaction that ran for hundreds of thousands of years! This natural nuclear reactor was discovered in 1972.
From Space
Most uranium on Earth was created in supernovas (exploding stars) long before our solar system formed. The uranium we mine today is essentially stardust that was part of the cloud that formed our solar system.
Energy Density
Uranium is incredibly energy-dense. The uranium fuel needed to power a nuclear reactor for one year would fit in a single soda can. To get the same energy from coal, you would need over 10,000 soda cans full of coal!
Named After a Planet
Uranium was named after the planet Uranus, which had been discovered just eight years earlier. This followed the tradition of naming new elements after recently discovered planets.





