What is Vapor Pressure - Definition, Examples, Quiz, FAQ, Trivia
Learn how liquids turn into gas and create pressure in closed spaces
What is Vapor Pressure?
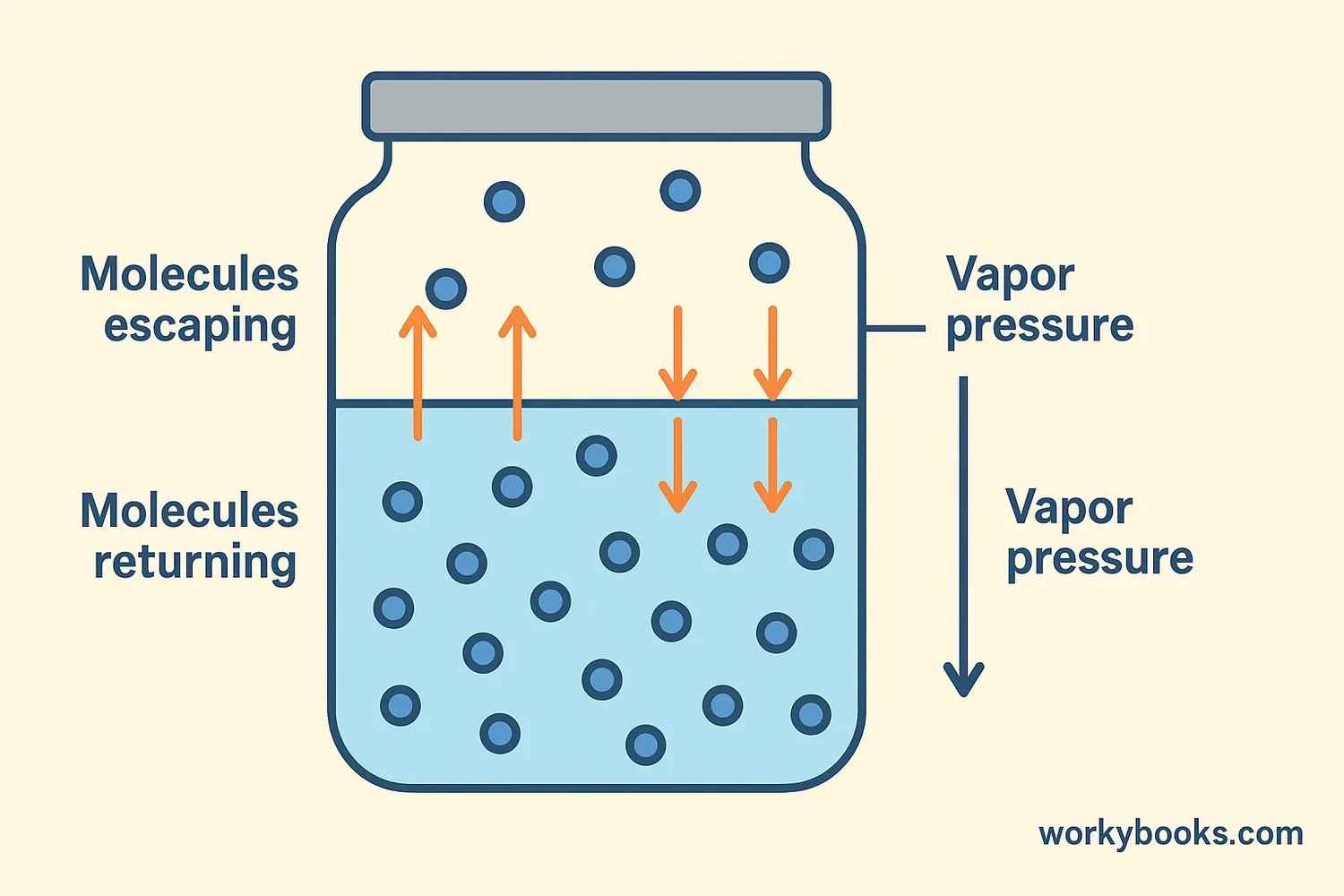
Vapor pressure is the pressure created by the gas (vapor) above a liquid in a closed container. When a liquid is in a closed space, some molecules escape from the liquid's surface and become gas. These gas molecules bounce around and push against the container's walls, creating vapor pressure.
Think of a soda bottle before you open it. The fizz (carbon dioxide gas) inside creates pressure - that's similar to vapor pressure! When the vapor pressure equals the pressure pushing down on the liquid, we call this equilibrium vapor pressure.
Science Fact!
Water at room temperature (25°C) has a vapor pressure of about 23.8 mmHg. That means the water vapor above the liquid pushes with this much force!
How Vapor Pressure Works
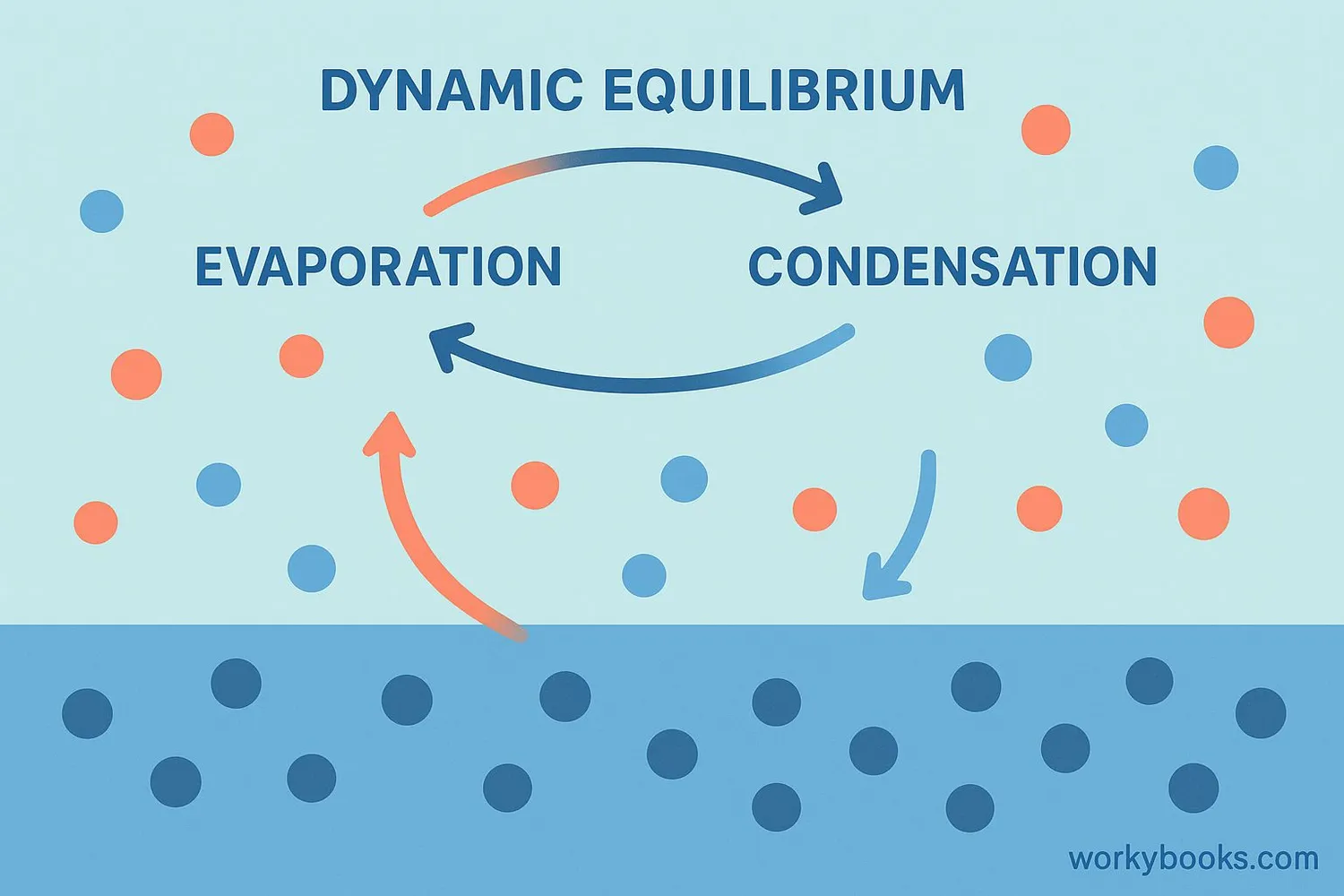
Vapor pressure develops through two important processes happening at the same time:
Evaporation
Molecules escape from the liquid surface into the air
Condensation
Gas molecules return to the liquid state
Equilibrium
When evaporation and condensation balance each other
Pressure Builds
Gas molecules create pressure on container walls
The saturation vapor pressure is the maximum pressure the vapor can reach at a given temperature. When this equals the surrounding pressure, the liquid boils! This explains why water boils at lower temperatures on mountaintops (where air pressure is lower).
Real-World Connection
This is why pressure cookers work - they increase pressure to raise the boiling point, cooking food faster!
Factors Affecting Vapor Pressure
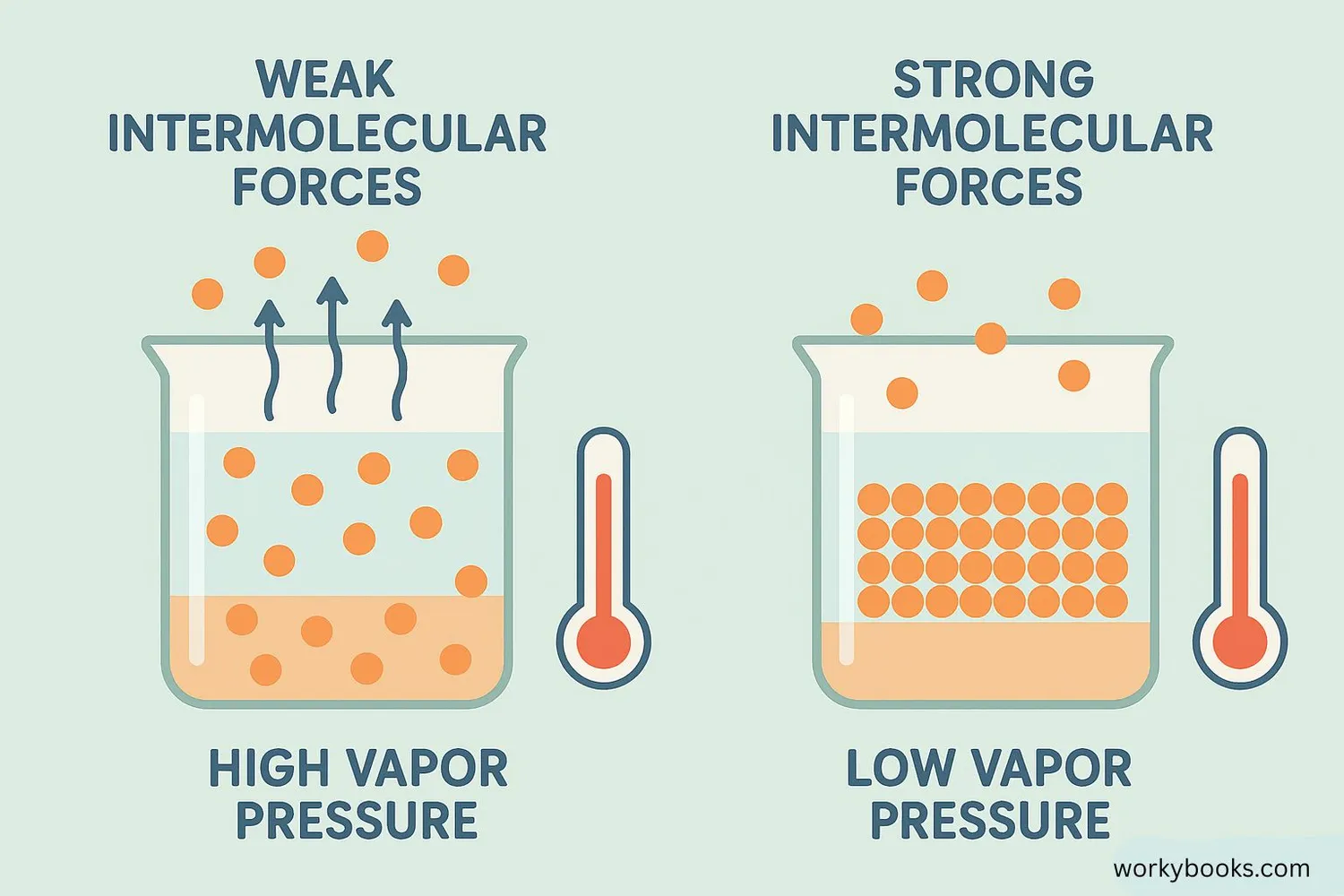
Several important factors determine how much vapor pressure a liquid will have:
Temperature
Higher temperature means more molecules have energy to escape, increasing vapor pressure
Intermolecular Forces
Stronger forces between molecules make it harder for them to escape, lowering vapor pressure
Nature of Liquid
Volatile liquids (like alcohol) evaporate easily and have higher vapor pressure
Raoult's Law explains how vapor pressure changes when liquids mix. The total vapor pressure depends on the mole fraction of each component. This is why adding salt to water lowers its vapor pressure - the salt molecules "get in the way" of water molecules trying to escape!
Vapor Pressure Quiz
Test your understanding with these questions about vapor pressure:
Frequently Asked Questions
Common questions students have about vapor pressure:
Vapor Pressure Science Facts
Discover some fascinating facts about vapor pressure:
Space Cooking
In space, water boils at body temperature (37°C) because there's no atmospheric pressure! This makes cooking very different in space stations.
Frog Breathing
Some frogs use vapor pressure differences to breathe through their skin. Their skin stays moist to allow oxygen to dissolve and diffuse into their blood.
Ice Cream Science
Adding salt to ice lowers water's vapor pressure, making the ice melt at a lower temperature. This creates the super-cold mixture needed to make ice cream!
Computer Cooling
Some high-performance computers use vapor pressure in heat pipes. Liquid evaporates at hot spots, carries heat away as vapor, then condenses to release it.





