Carboxylic Acids - Definition, Examples, Quiz, FAQ, Trivia
Discover the special acids that give foods their flavor and help make everyday products!
What Are Carboxylic Acids?
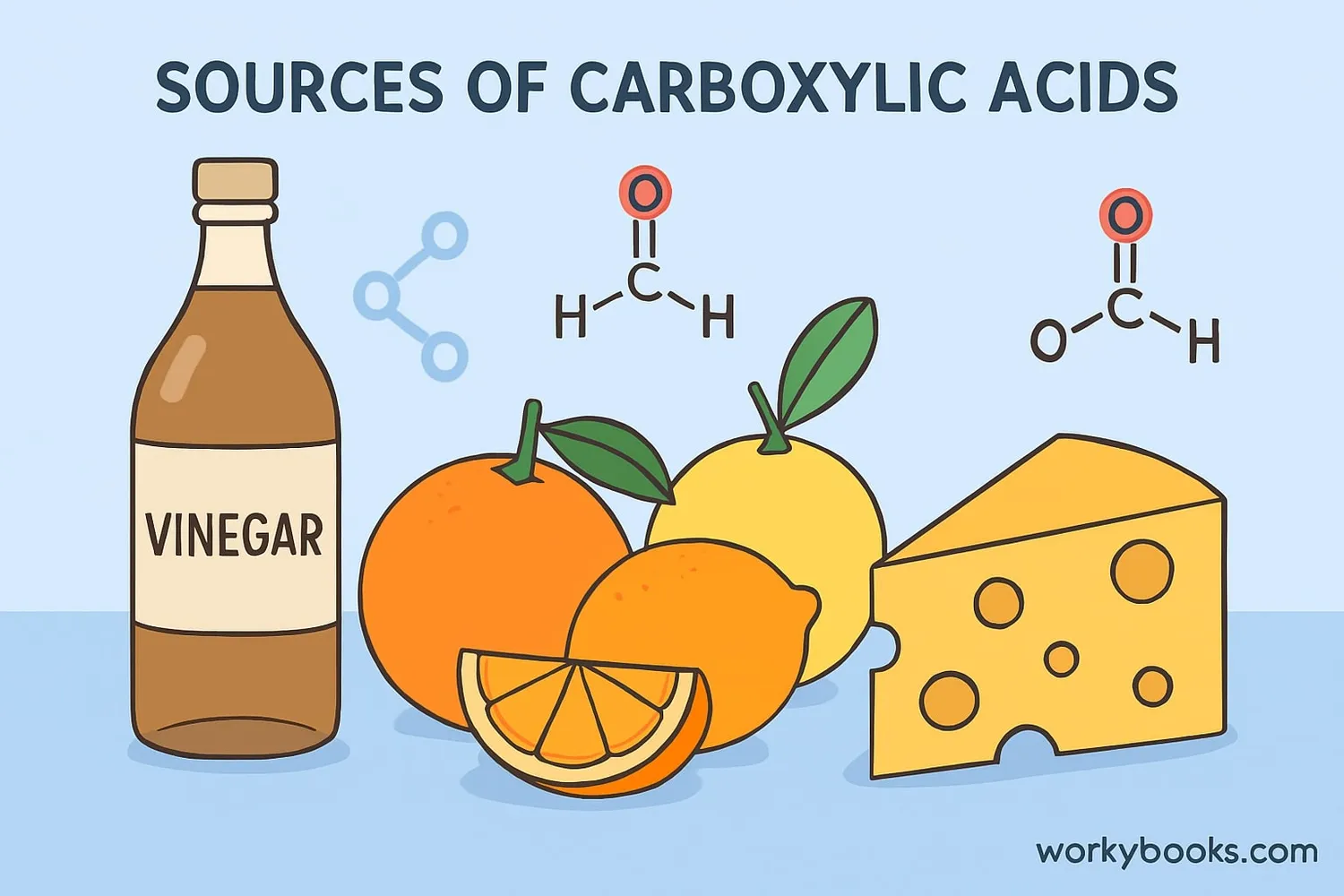
Carboxylic acids are a special group of organic compounds that contain a carboxyl functional group (-COOH). They're found in many things around us! The sour taste in vinegar comes from acetic acid, and the tangy flavor in lemons comes from citric acid - both carboxylic acids.
These acids are made of carbon, hydrogen, and oxygen atoms. The "carboxyl" part of their name comes from "carbon" and "oxygen." They're important building blocks in chemistry and nature, helping create everything from flavors in food to materials for clothes.
Chemical Fact!
The simplest carboxylic acid is formic acid, which is found in ant stings and gives them their painful bite!
Chemical Structure
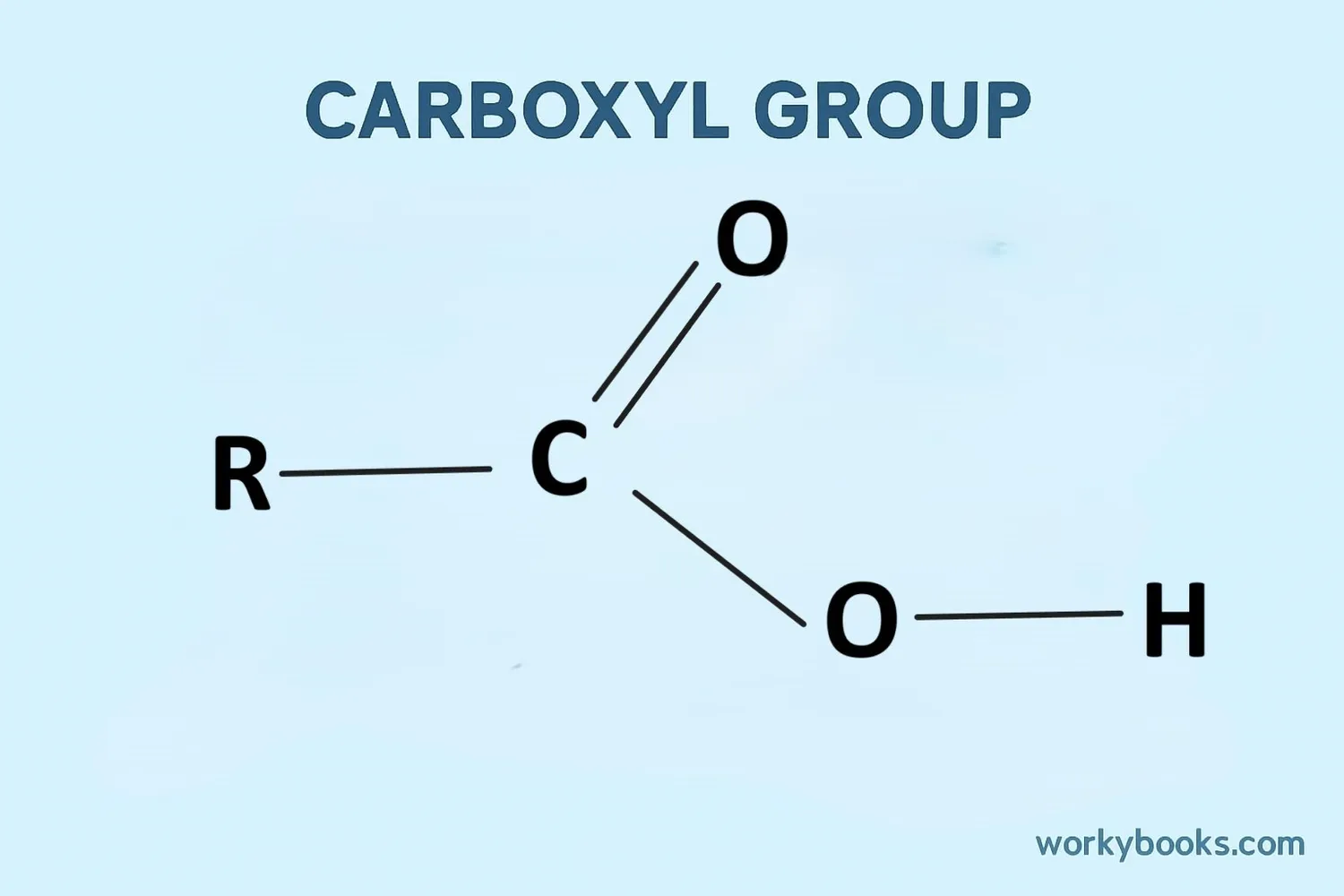
The special part of carboxylic acids is the carboxyl group, which looks like this: -COOH. This group has:
• A carbon atom (C)
• Double-bonded to an oxygen atom (O)
• Single-bonded to a hydroxyl group (OH)
The general formula for carboxylic acids is R-COOH, where "R" can be different groups of atoms. For example:
• Acetic acid: CH₃-COOH (vinegar)
• Formic acid: H-COOH (ant stings)
• Butyric acid: CH₃CH₂CH₂-COOH (butter)
Bonding Fact!
The double bond between carbon and oxygen in the carboxyl group makes these molecules polar, which affects how they interact with other substances.
Properties of Carboxylic Acids
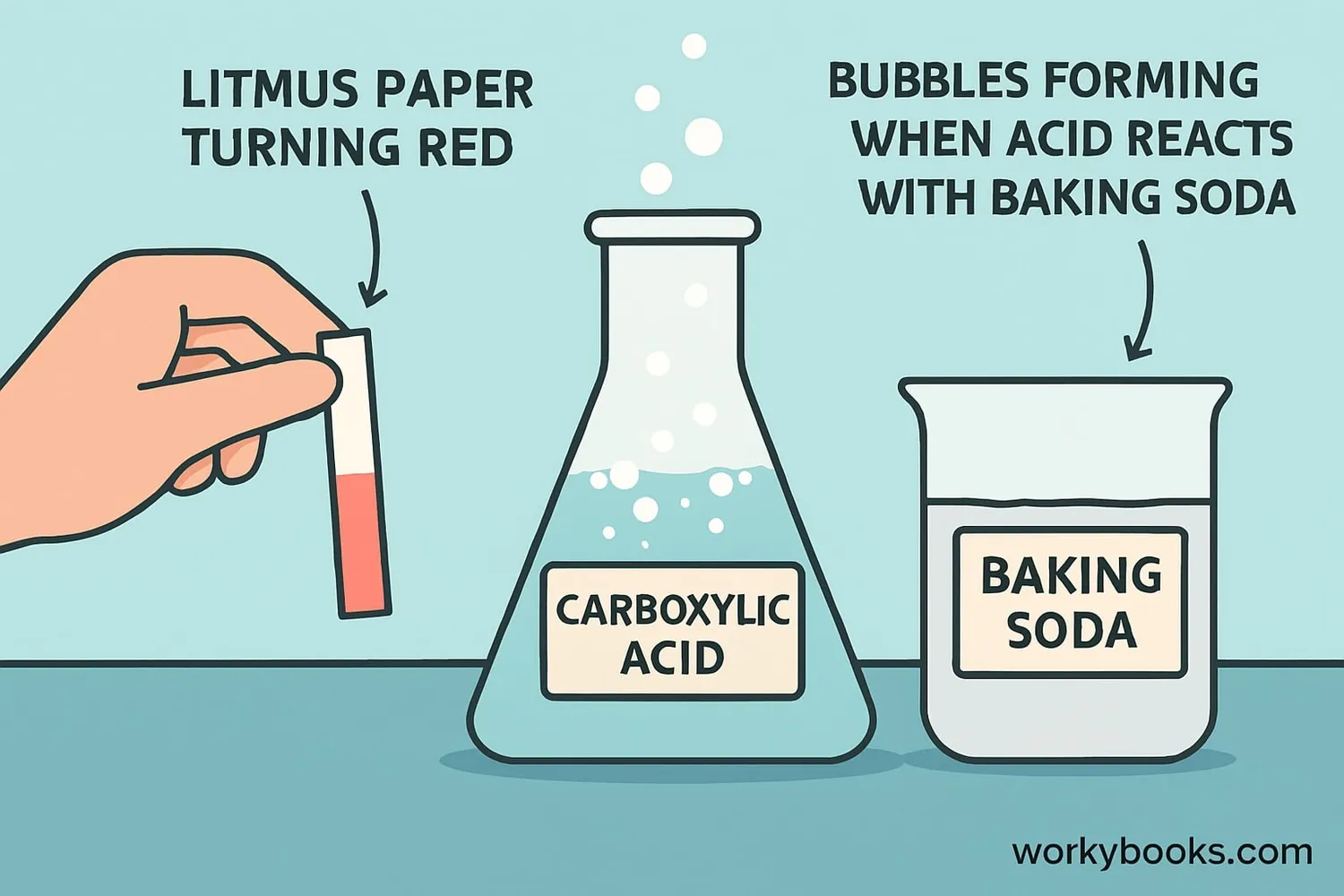
Carboxylic acids have several special properties that make them useful and interesting:
Sour Taste
Many carboxylic acids taste sour, like vinegar or lemon juice
Acidic Nature
They turn blue litmus paper red and have pH less than 7
Strong Odors
Some have strong smells (vinegar, sweaty socks)
Hydrogen Bonding
They form strong bonds with water, making them soluble
Higher Boiling Points
They boil at higher temperatures than similar molecules
When carboxylic acids react with bases, they form salts and water in a process called neutralization. For example, vinegar (acetic acid) reacts with baking soda (sodium bicarbonate) to make sodium acetate, water, and carbon dioxide gas - that's why you see bubbles!
Common Carboxylic Acids
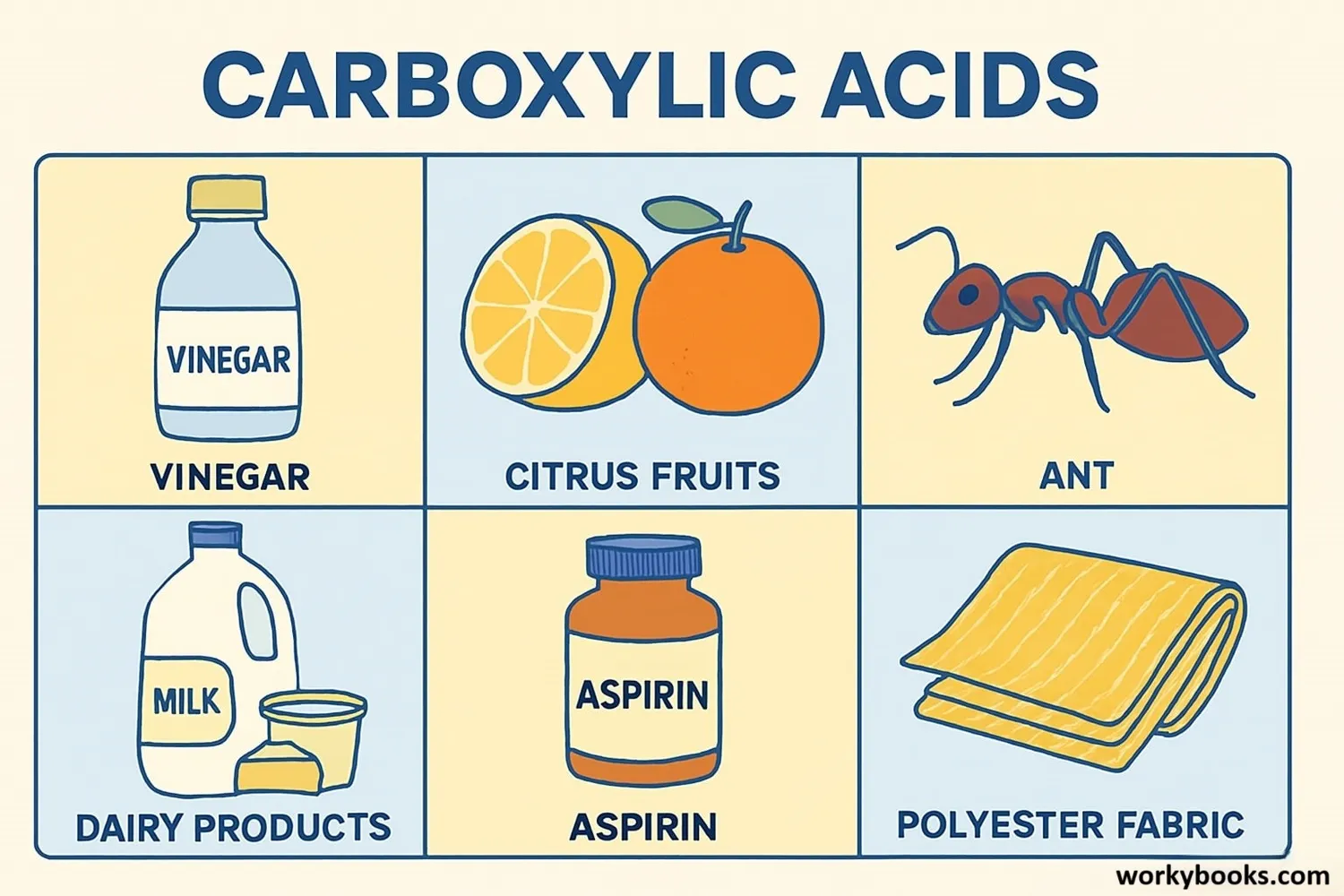
Carboxylic acids are all around us! Here are some common examples you might know:
Acetic Acid
Found in vinegar, used in cooking and cleaning
Citric Acid
Found in citrus fruits, used in foods and cleaners
Formic Acid
Found in ant stings, used in textiles and leather
Butyric Acid
Gives butter its smell, found in dairy products
Salicylic Acid
Used in aspirin and acne treatments
These acids are also important in making:
• Plastics and polymers
• Medicines like aspirin
• Food preservatives
• Perfumes and flavorings
• Fabrics like polyester
Without carboxylic acids, many everyday products wouldn't exist!
Carboxylic Acids Quiz
Test your knowledge with this quiz! Answer all 5 questions to see how much you've learned about carboxylic acids.
Frequently Asked Questions
Here are answers to some common questions about carboxylic acids:
Fun Carboxylic Acid Trivia
Discover some amazing facts about carboxylic acids!
Ancient Origins
Vinegar (dilute acetic acid) has been used for over 10,000 years! Ancient Babylonians used it as a preservative and condiment as early as 5000 BC.
Formic Acid Warriors
Some ants can spray formic acid up to 20 cm (8 inches) to defend themselves! This acid got its name from the Latin word for ant, "formica."
Fatty Acids
Long-chain carboxylic acids are called fatty acids. They're essential building blocks for fats in our bodies and in foods. Your body can make some but not all of them!
Space Acids
Astronomers have found carboxylic acids in space! Formic acid was detected in interstellar gas clouds, showing these compounds form naturally throughout the universe.





