Gamma Decay - Definition, Examples, Quiz, FAQ, Trivia
Learn about gamma radiation and how atoms release energy
What is Gamma Decay?
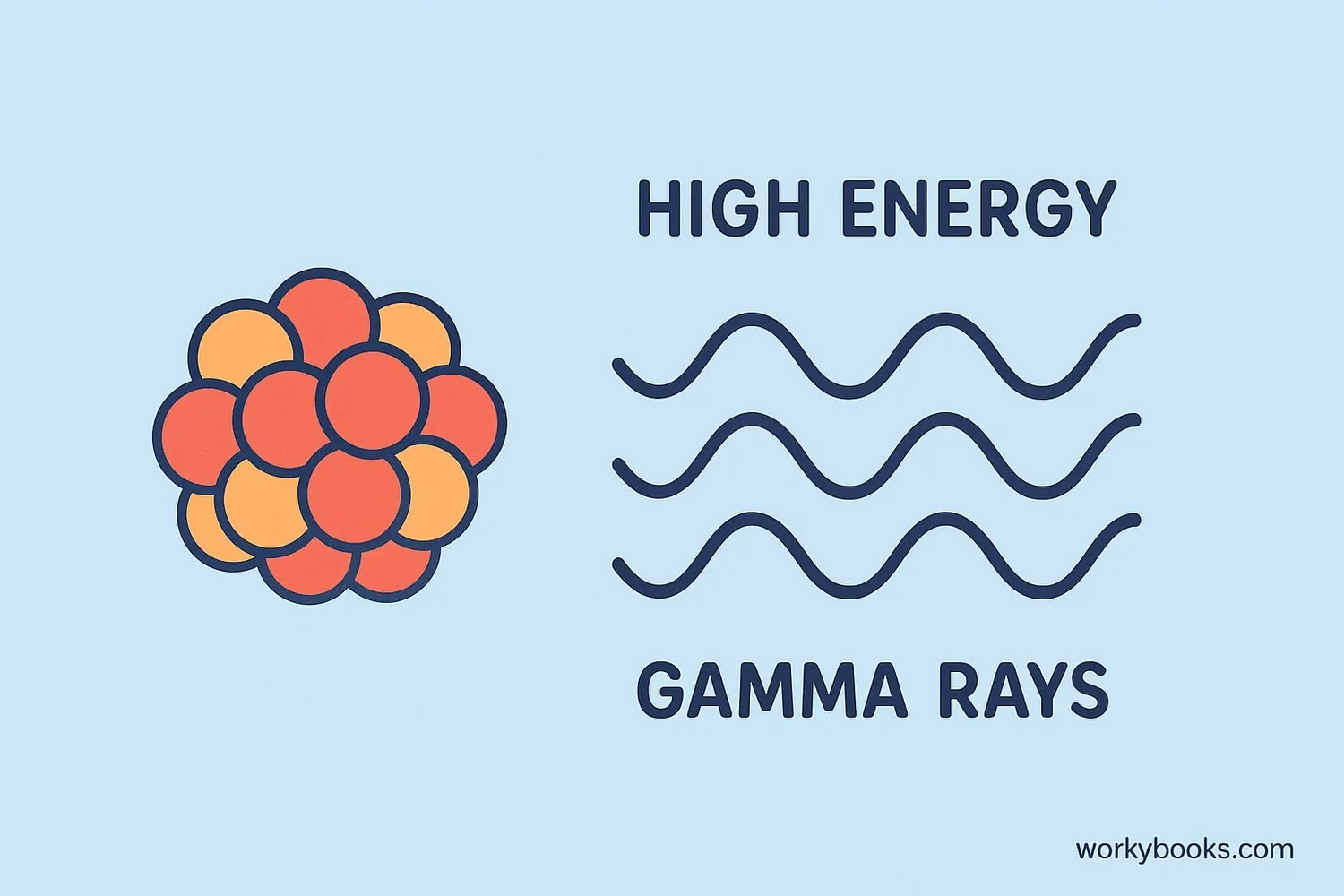
Gamma decay is a type of radioactive decay where an atomic nucleus releases excess energy in the form of gamma rays. Unlike other forms of radioactive decay, gamma decay doesn't change the composition of the atom—it only releases energy.
Think of it like this: when an atom has too much energy, it needs to "cool down" by releasing that extra energy. The energy is released as gamma radiation, which is a form of electromagnetic waves, similar to light but with much higher energy.
Science Fact!
Gamma rays are the most energetic form of light in the universe, with wavelengths shorter than atoms!
How Gamma Decay Works
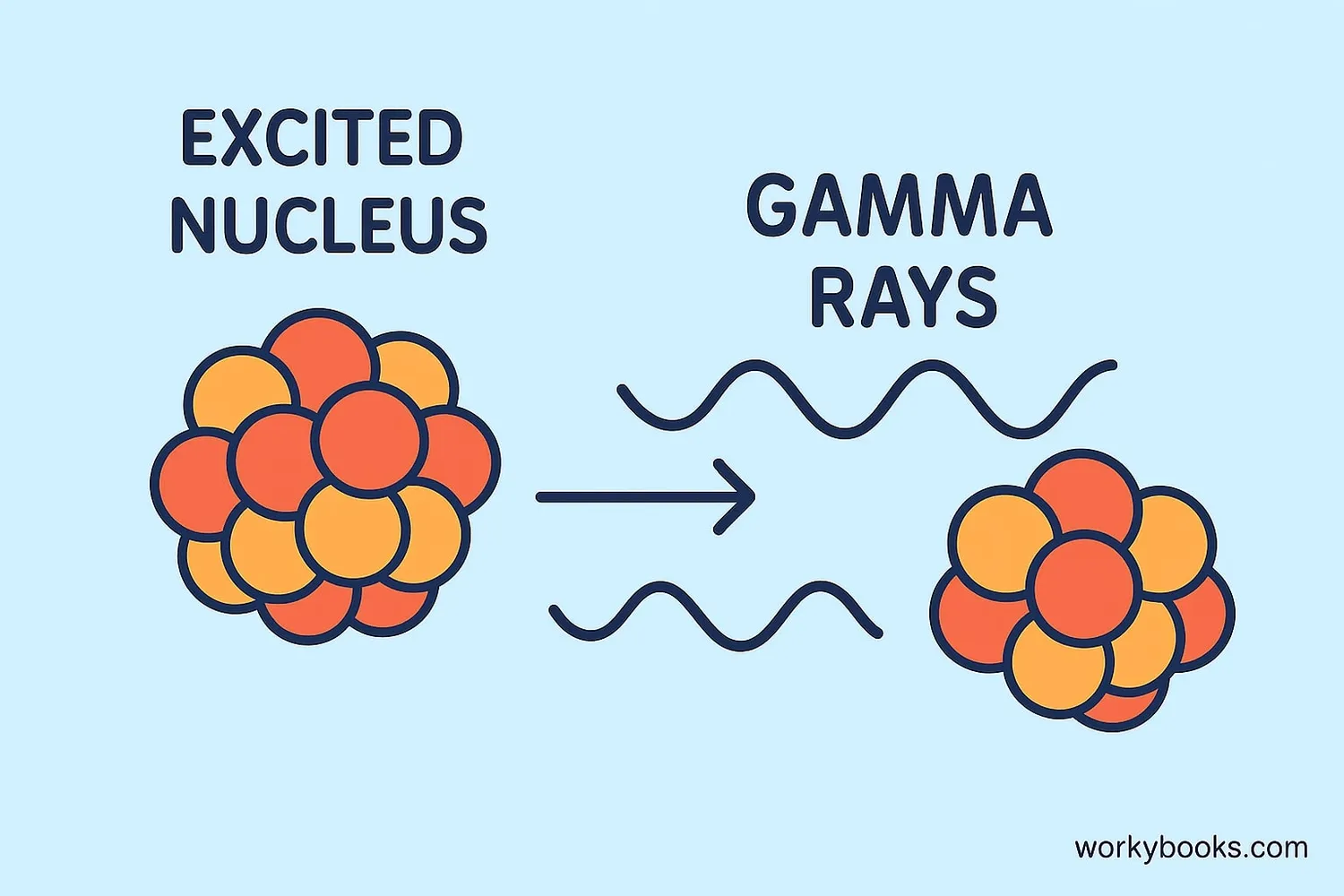
Gamma decay occurs after other types of radioactive decay have happened. When an atom undergoes alpha or beta decay, it's often left in an "excited" state with extra energy. To return to a stable state, it releases this energy as gamma radiation.
Initial Decay
Atom undergoes alpha or beta decay
Excited State
Nucleus is left with excess energy
Energy Release
Nucleus emits gamma rays to stabilize
Stable State
Atom returns to its lowest energy state
The key thing to remember about gamma decay is that it only changes the energy of the nucleus, not its composition. The number of protons and neutrons stays the same—only the energy level changes.
Gamma Ray Properties!
Gamma rays have no mass and no electrical charge. They're pure energy that travels at the speed of light!
Why Gamma Decay is Important
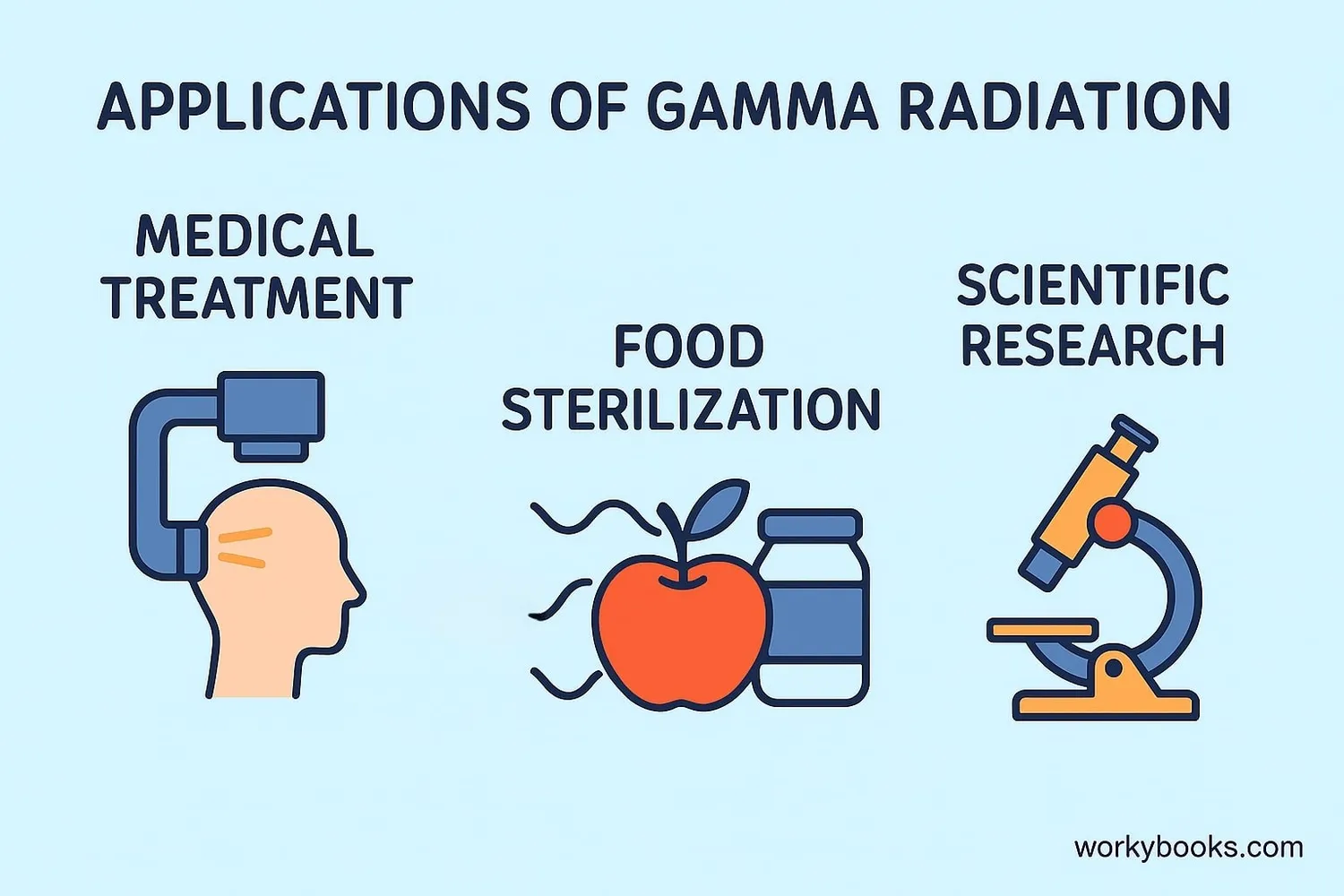
Understanding gamma decay helps scientists in many important fields. Here's why it matters:
Medical Applications
Used in radiation therapy to treat cancer and sterilize medical equipment
Food Preservation
Kills bacteria and extends shelf life of food products
Scientific Research
Helps scientists study atomic structure and nuclear processes
Gamma decay also helps us understand:
• How stars produce energy
• How elements are formed in the universe
• How to safely handle radioactive materials
• How to detect and measure radiation
By studying gamma decay, scientists can develop new technologies and improve safety measures for using radioactive materials.
Gamma Decay Quiz
Test your knowledge about gamma decay with this quiz. Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about gamma decay:
Interesting Facts About Gamma Decay
Discover some fascinating information about gamma decay and radiation:
Incredible Energy
Gamma rays have the highest energy of any wave in the electromagnetic spectrum. A single gamma ray photon can have millions of times more energy than a photon of visible light!
Cosmic Origins
Some of the most powerful gamma-ray sources in the universe are gamma-ray bursts, which release more energy in seconds than our sun will emit in its entire 10-billion-year lifetime!
Historical Discovery
French chemist Paul Villard discovered gamma radiation in 1900 while studying radiation from radium. The name "gamma" was given by Ernest Rutherford in 1903.
Shielding Challenge
Stopping gamma rays requires thick barriers of lead or concrete. Unlike alpha and beta radiation, which can be stopped by paper or clothing, gamma rays require much denser materials for protection.





