Oxidation Reaction - Definition, Examples, Quiz, FAQ, Trivia
Understanding How Substances Gain Oxygen and Lose Electrons
What is Oxidation?
Oxidation is a chemical reaction where a substance loses electrons or gains oxygen. It's one of the most common chemical processes we see in everyday life! When metals rust, when fruits turn brown, or when fire burns - these are all examples of oxidation reactions.
Originally, oxidation specifically meant a substance combining with oxygen. But today, we understand it more broadly as any chemical reaction where atoms, ions, or molecules lose electrons.
Science Fact!
The term "oxidation" comes from the word "oxygen," which was discovered by scientist Carl Wilhelm Scheele. Oxygen is involved in many common oxidation reactions!
Redox Reactions
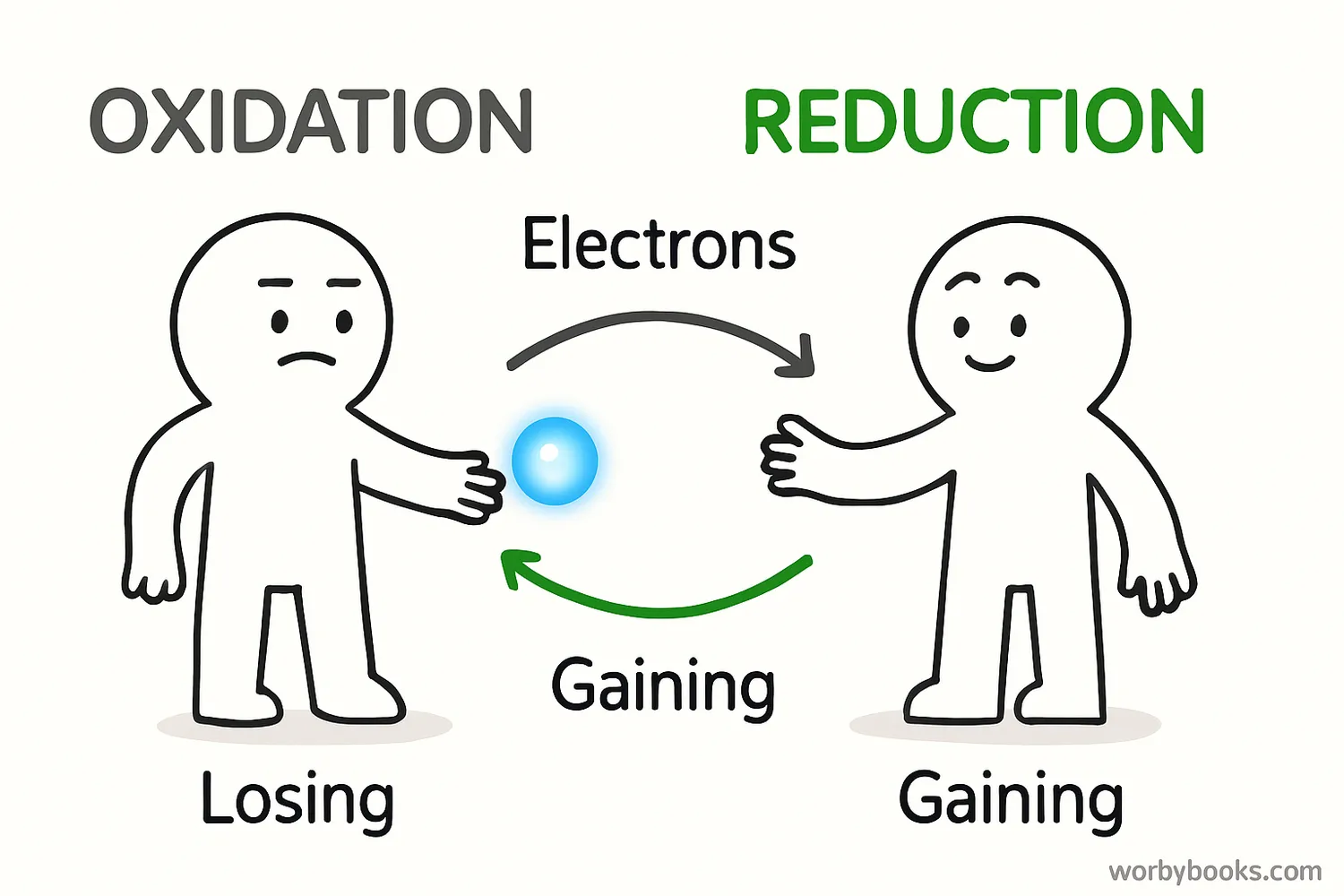
Oxidation never happens alone! It's always paired with a process called reduction. Together, they form redox reactions (short for reduction-oxidation reactions).
In a redox reaction:
Oxidation
The substance that loses electrons is oxidized
Reduction
The substance that gains electrons is reduced
A helpful way to remember this is with the mnemonic: OIL RIG - Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons).
(of electrons)
Oxidizing & Reducing Agents
In redox reactions, we have special names for the substances that cause oxidation or reduction:
| Agent Type | Definition | What It Does | Examples |
|---|---|---|---|
| Oxidizing Agent | Accepts electrons | Causes oxidation in another substance | Oxygen, chlorine, hydrogen peroxide |
| Reducing Agent | Donates electrons | Causes reduction in another substance | Carbon, hydrogen, metals like sodium |
Remember: The oxidizing agent gets reduced (gains electrons), and the reducing agent gets oxidized (loses electrons). It might seem confusing at first, but with practice, it becomes clearer!
Oxidation Number
Scientists use oxidation numbers to keep track of electron transfers in redox reactions. An oxidation number is a hypothetical charge that an atom would have if all bonds were ionic.
Here are some simple rules for determining oxidation numbers:
Elemental Form
Atoms in elemental form have oxidation number 0 (e.g., Na, O₂, H₂)
Monatomic Ions
Equal to the ion's charge (e.g., Na⁺ = +1, Cl⁻ = -1)
Oxygen
Usually -2 (except in peroxides where it's -1)
Hydrogen
Usually +1 (except in metal hydrides where it's -1)
In a redox reaction, when an atom's oxidation number increases, it's being oxidized. When it decreases, it's being reduced.
Examples of Oxidation
Oxidation reactions are happening all around us every day. Here are some common examples:
| Example | What's Oxidized | Oxidizing Agent | Chemical Equation |
|---|---|---|---|
| Rusting Iron | Iron metal | Oxygen | 4Fe + 3O₂ → 2Fe₂O₃ |
| Fruit Browning | Compounds in fruit | Oxygen | Enzymatic oxidation |
| Combustion | Fuel (wood, gas) | Oxygen | CH₄ + 2O₂ → CO₂ + 2H₂O |
| Battery Operation | Zinc (in alkaline batteries) | Manganese dioxide | Zn + 2MnO₂ → ZnO + Mn₂O₃ |
Not all oxidation is destructive! Our bodies use controlled oxidation reactions to convert food into energy through cellular respiration. This shows how oxidation can be both helpful and harmful depending on the context.
Oxidation Reaction Quiz
Test your knowledge with this quiz! Answer all 5 questions to see how much you've learned about oxidation reactions.
Frequently Asked Questions
Here are answers to some common questions about oxidation reactions:
Science Facts About Oxidation
Discover some fascinating facts about oxidation reactions!
Cost of Rust
Oxidation of iron (rusting) costs the U.S. economy approximately $400 billion annually in replacement and repair costs for infrastructure, vehicles, and equipment.
Breathing and Oxidation
The oxygen you breathe is used in oxidation reactions in your cells to release energy from food. An average person uses about 550 liters of pure oxygen each day for these reactions!
Rocket Fuel
Rocket launches use powerful oxidation reactions. The space shuttle's main engines burned liquid hydrogen (fuel) with liquid oxygen (oxidizer) at temperatures over 3,300°C (6,000°F)!
Apple Browning
When you slice an apple and it turns brown, that's enzymatic oxidation. The enzyme polyphenol oxidase reacts with oxygen in the air, creating melanin—the same pigment that colors human skin!





