Synthesis Reactions - Definition, Examples, Quiz, FAQ, Trivia
Learn how chemicals combine to form new substances
What is a Synthesis Reaction?
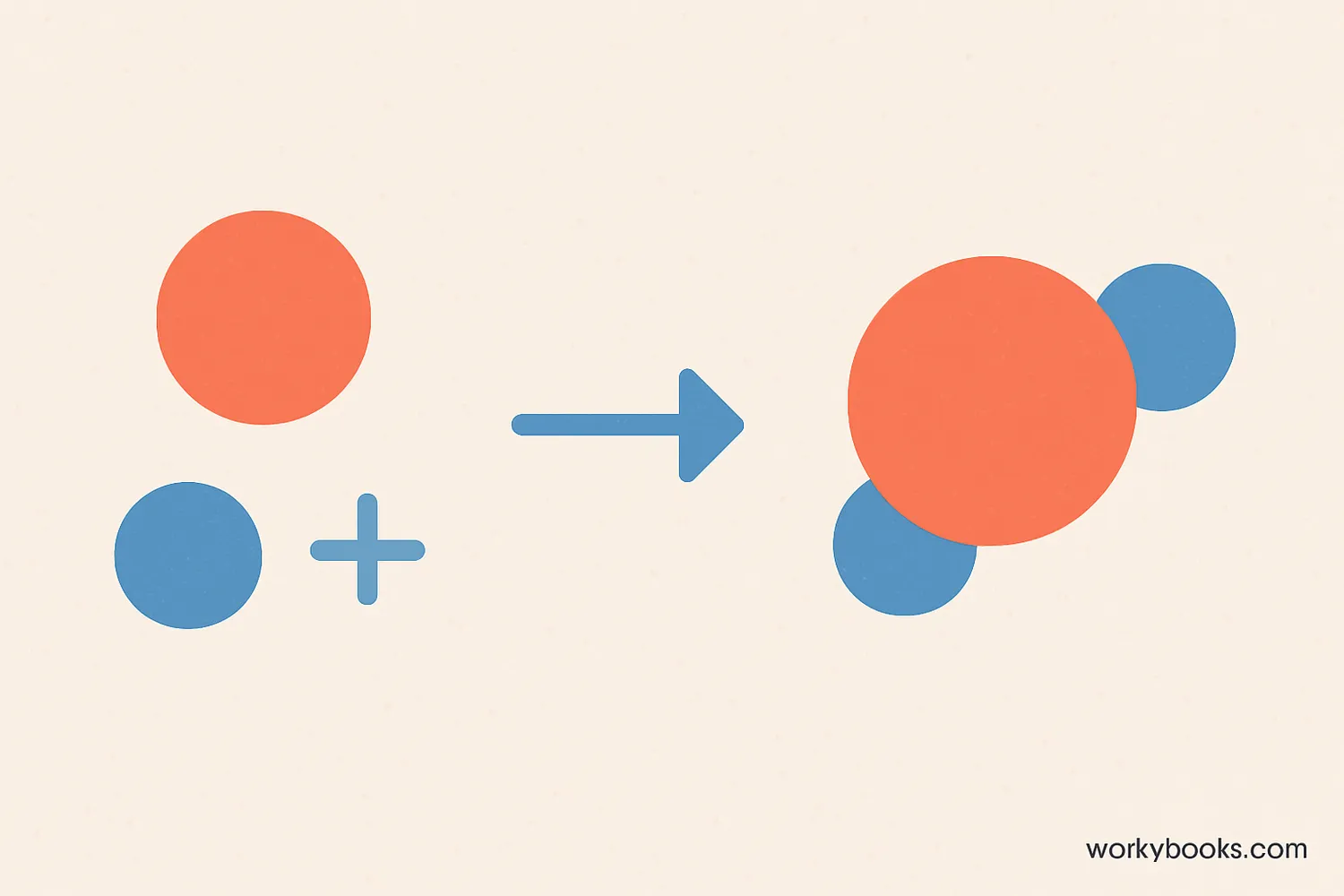
A synthesis reaction is a type of chemical reaction where two or more simple substances combine to form a more complex product. It's like building with LEGO blocks - you start with individual pieces and connect them to create something new!
The general formula for a synthesis reaction is: A + B → AB
In this reaction, elements or compounds (A and B) join together to form a new compound (AB). Synthesis reactions are also called combination reactions or anabolic reactions.
Key Fact
Synthesis reactions often require energy to occur, which makes them endergonic reactions. This means they absorb energy from their surroundings.
How Synthesis Reactions Work
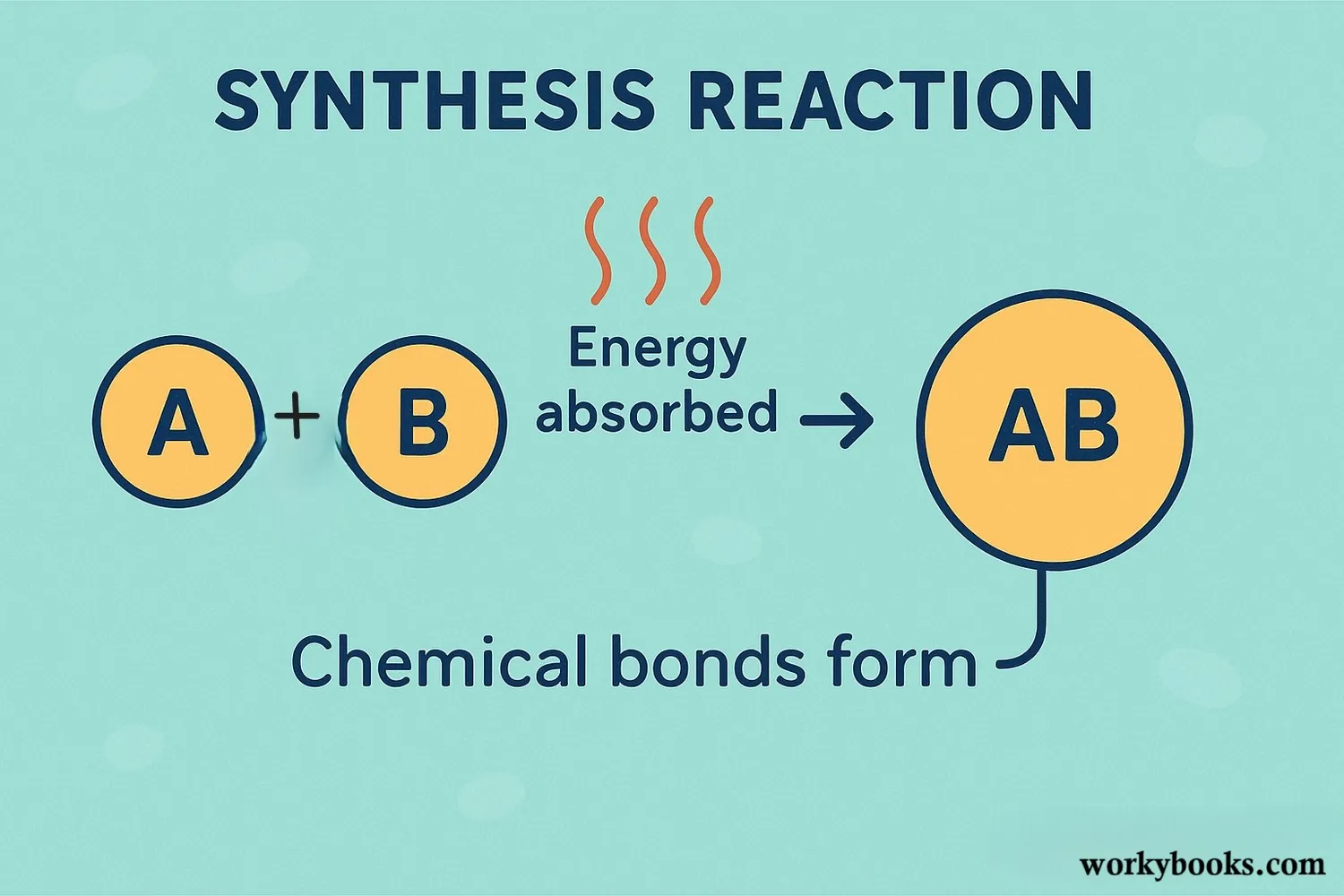
Synthesis reactions involve the formation of new chemical bonds between atoms or molecules. Here's what happens during these reactions:
Reactants Approach
Two or more substances come close together
Energy Absorption
Energy is absorbed to break existing bonds
Bond Formation
New chemical bonds form between atoms
Product Formation
A new compound is created with different properties
The energy needed for synthesis reactions can come from different sources like heat, light, or electricity. In living organisms, special proteins called enzymes help synthesis reactions happen more efficiently.
Reaction Energy
Most synthesis reactions are endothermic, meaning they absorb heat energy from their surroundings. This is why some chemical reactions feel cold to the touch!
Examples of Synthesis Reactions
Synthesis reactions are all around us! Here are some common examples you might encounter:
Water Formation
2H₂ + O₂ → 2H₂O
Hydrogen gas and oxygen gas combine to form water
Photosynthesis
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
Plants use sunlight to create glucose from CO₂ and water
Rust Formation
4Fe + 3O₂ → 2Fe₂O₃
Iron reacts with oxygen to form iron oxide (rust)
Other examples include:
• Protein synthesis in your body where amino acids join to form proteins
• Ammonia production where nitrogen and hydrogen combine (N₂ + 3H₂ → 2NH₃)
• Baking soda formation from sodium carbonate, water, and carbon dioxide
• Metal oxide formation when metals react with oxygen
Importance of Synthesis Reactions
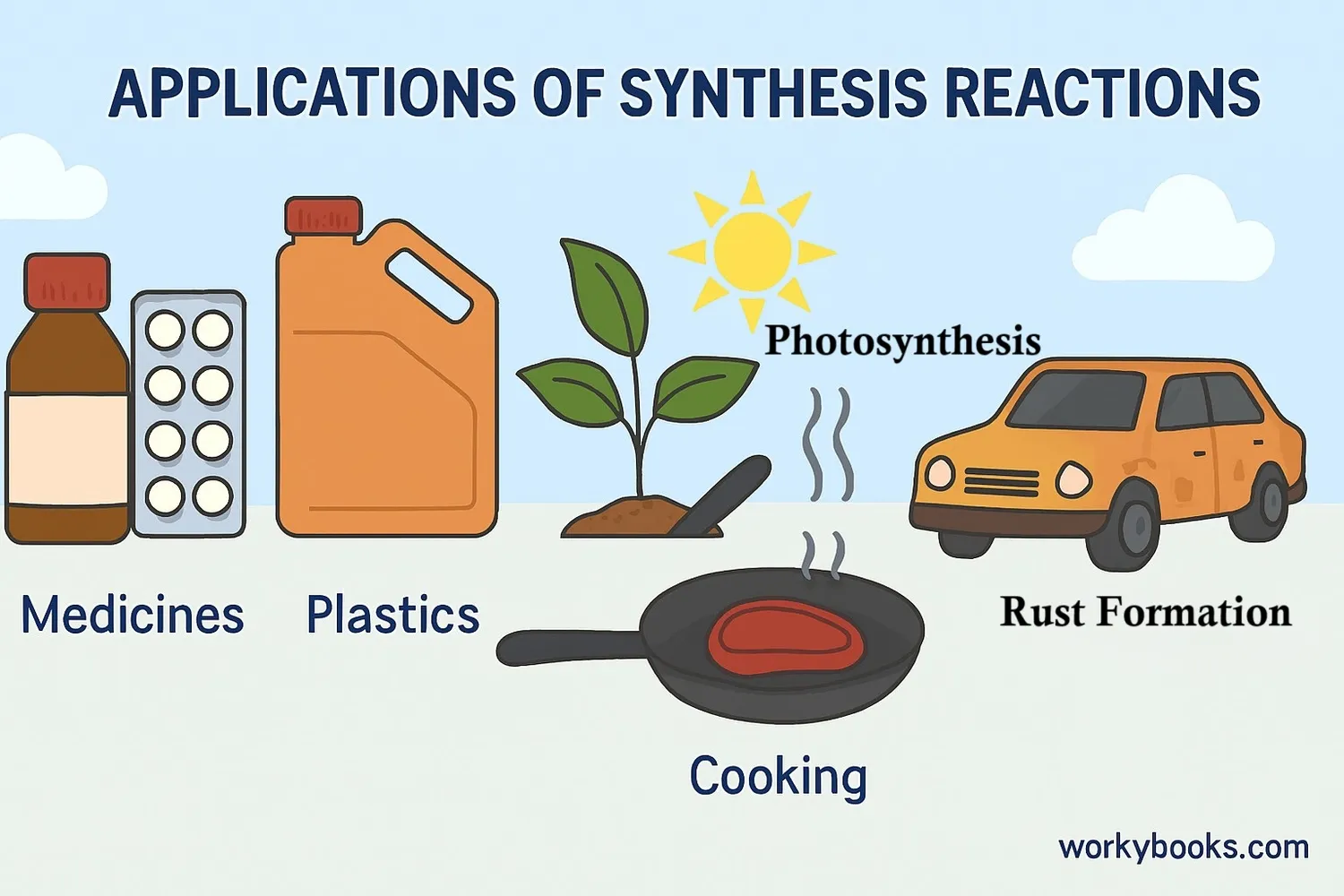
Synthesis reactions are fundamental to many processes in nature, industry, and our bodies. Here's why they're so important:
Industrial Applications
Used to create medicines, plastics, fertilizers, and many other products
Biological Processes
Essential for photosynthesis, protein synthesis, and DNA replication
Energy Storage
Help store energy in chemical bonds for later use
Without synthesis reactions, we wouldn't have:
• The food we eat (created through photosynthesis)
• The materials for our homes and clothes
• Medicines to keep us healthy
• The ability to grow and repair our bodies
Understanding synthesis reactions helps scientists develop new materials, create sustainable energy solutions, and understand how living organisms function.
Synthesis Reaction Quiz
Test your knowledge about synthesis reactions with this quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about synthesis reactions:
Fun Synthesis Reaction Trivia
Discover some amazing facts about synthesis reactions!
Industrial Impact
The Haber process, which synthesizes ammonia from nitrogen and hydrogen, is one of the most important industrial synthesis reactions. It produces fertilizer that helps grow food for about half the world's population!
Protein Power
Your body performs about 10,000 different synthesis reactions every second to build proteins! Ribosomes in your cells act as tiny factories where amino acids are linked together to form proteins.
Historical Discovery
The first recognized synthesis reaction was performed by Joseph Priestley in 1774 when he synthesized water by burning hydrogen in air. This helped prove that water is a compound, not an element.
Energy Storage
Photosynthesis is the most important synthesis reaction on Earth. It stores solar energy in chemical bonds of glucose molecules, providing energy for nearly all life on our planet.





