Allotropes - Definition, Examples, Quiz, FAQ, Trivia
Discover how carbon can be both pencil lead and sparkling diamonds!
What are Allotropes?
Allotropes are different forms of the same element! Imagine having the exact same building blocks but arranging them differently to create completely different materials. That's what happens with allotropes!
For example, carbon atoms can arrange themselves in different patterns to create:
- Diamond (hard and sparkling)
- Graphite (soft and dark)
- Graphene (super thin and strong)
The word "allotrope" comes from Greek words meaning "other" and "form." It's like the element is wearing different costumes!
Science Fact!
Not all elements have allotropes - only some like carbon, oxygen, phosphorus, and sulfur can form different structures with the same atoms!
Amazing Carbon Allotropes
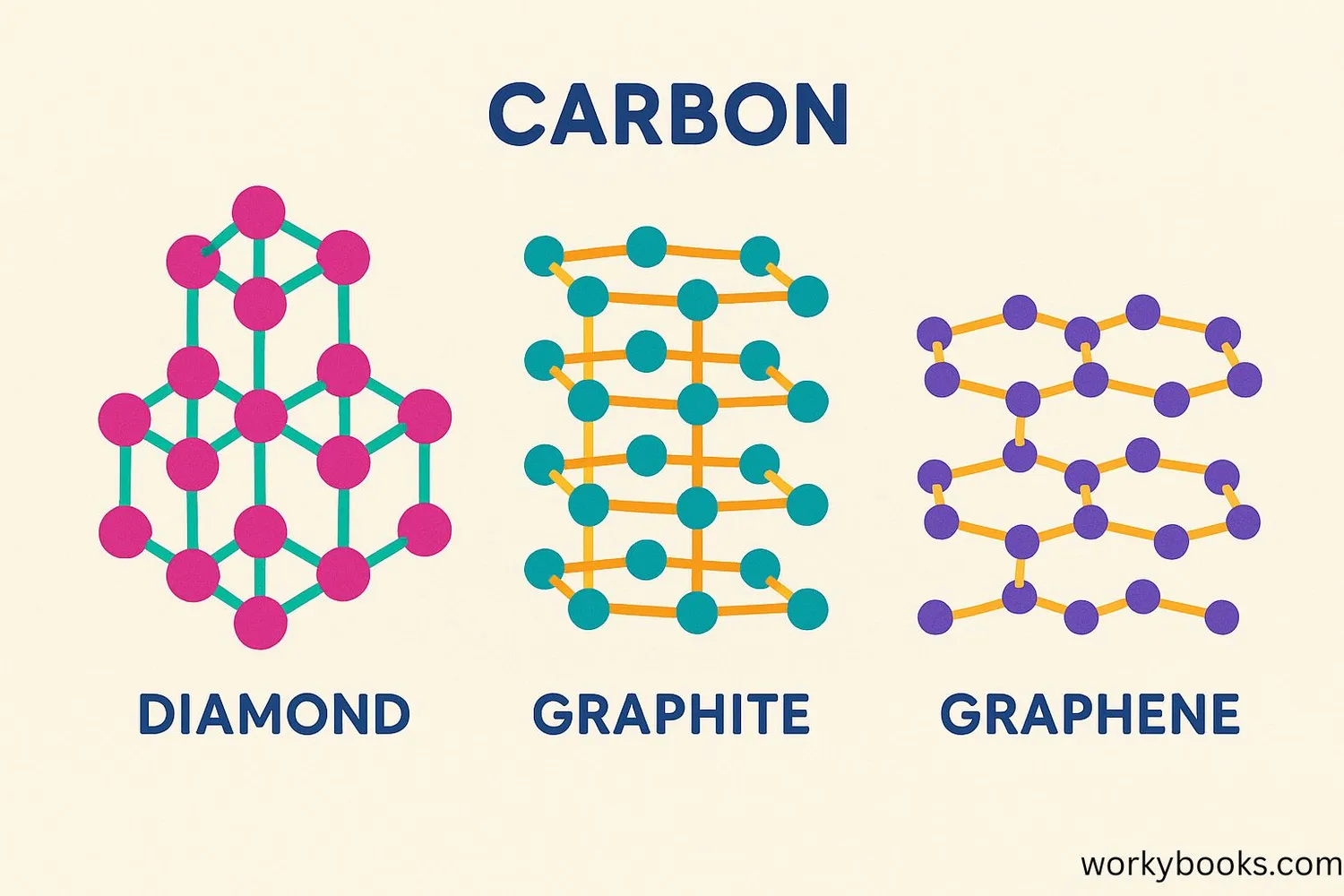
Carbon is the champion of allotropes! It can form more allotropes than any other element. Let's look at three famous carbon allotropes:
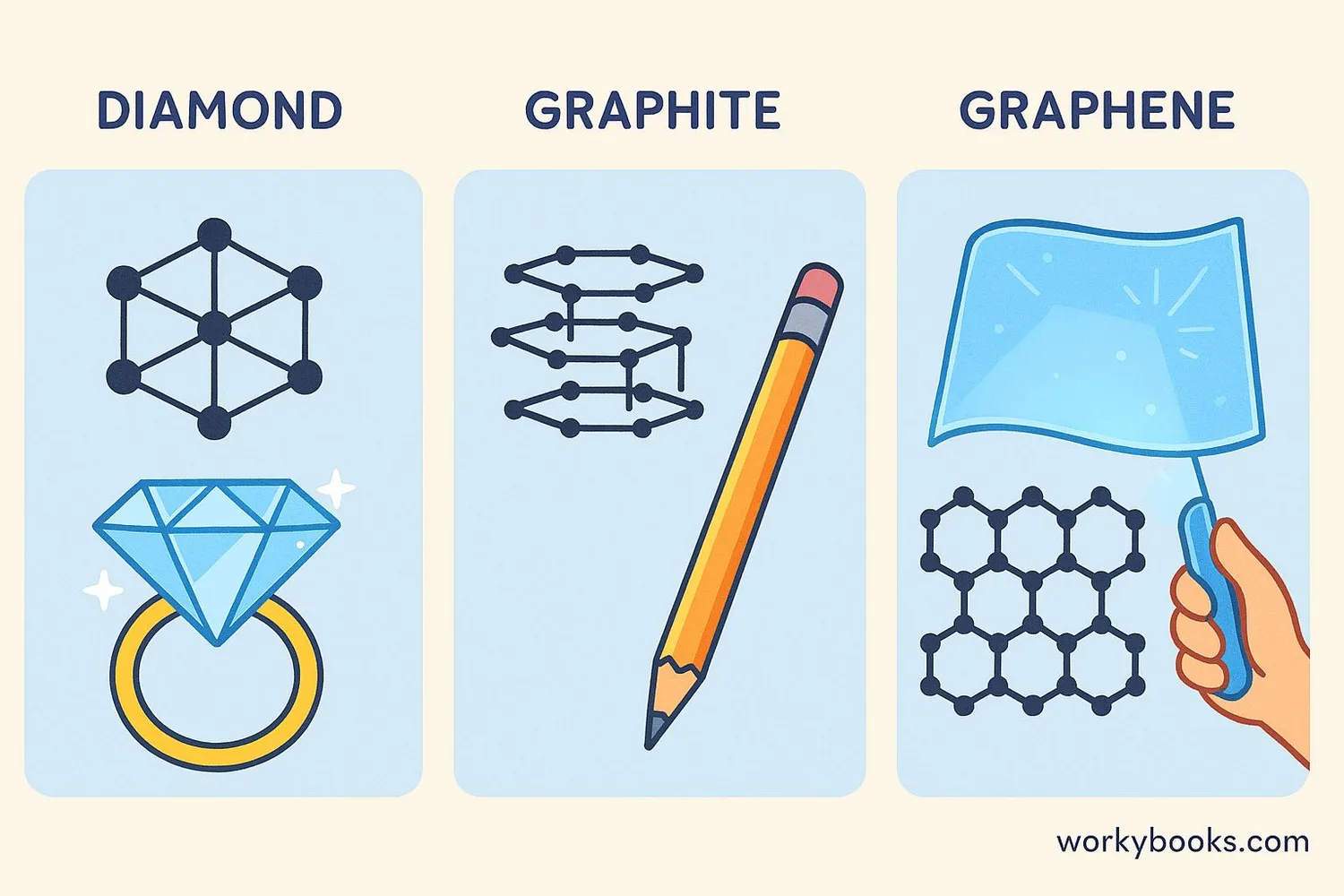
Diamond
Each carbon atom connects to four others in a strong 3D pattern
Properties: Hardest natural material, sparkly, great for jewelry and cutting tools
Graphite
Carbon atoms form flat sheets that slide over each other
Properties: Soft, dark, conducts electricity, used in pencils and lubricants
Graphene
A single layer of graphite atoms in a honeycomb pattern
Properties: Thinnest material, super strong, conducts electricity better than copper
How can the same carbon atoms create such different materials? It all comes down to chemical bonding and molecular arrangements. The way atoms connect to each other changes everything about the material's properties!
Did You Know?
Scientists are constantly discovering new carbon allotropes! Fullerene (shaped like a soccer ball) was only discovered in 1985, and graphene in 2004.
How Allotropes Form
Allotropes form under different conditions. The arrangement of atoms depends on:
Temperature
Heat energy affects how atoms move and connect
Pressure
High pressure can force atoms into different arrangements
Chemical Environment
Other elements present can influence atomic arrangements
For carbon allotropes:
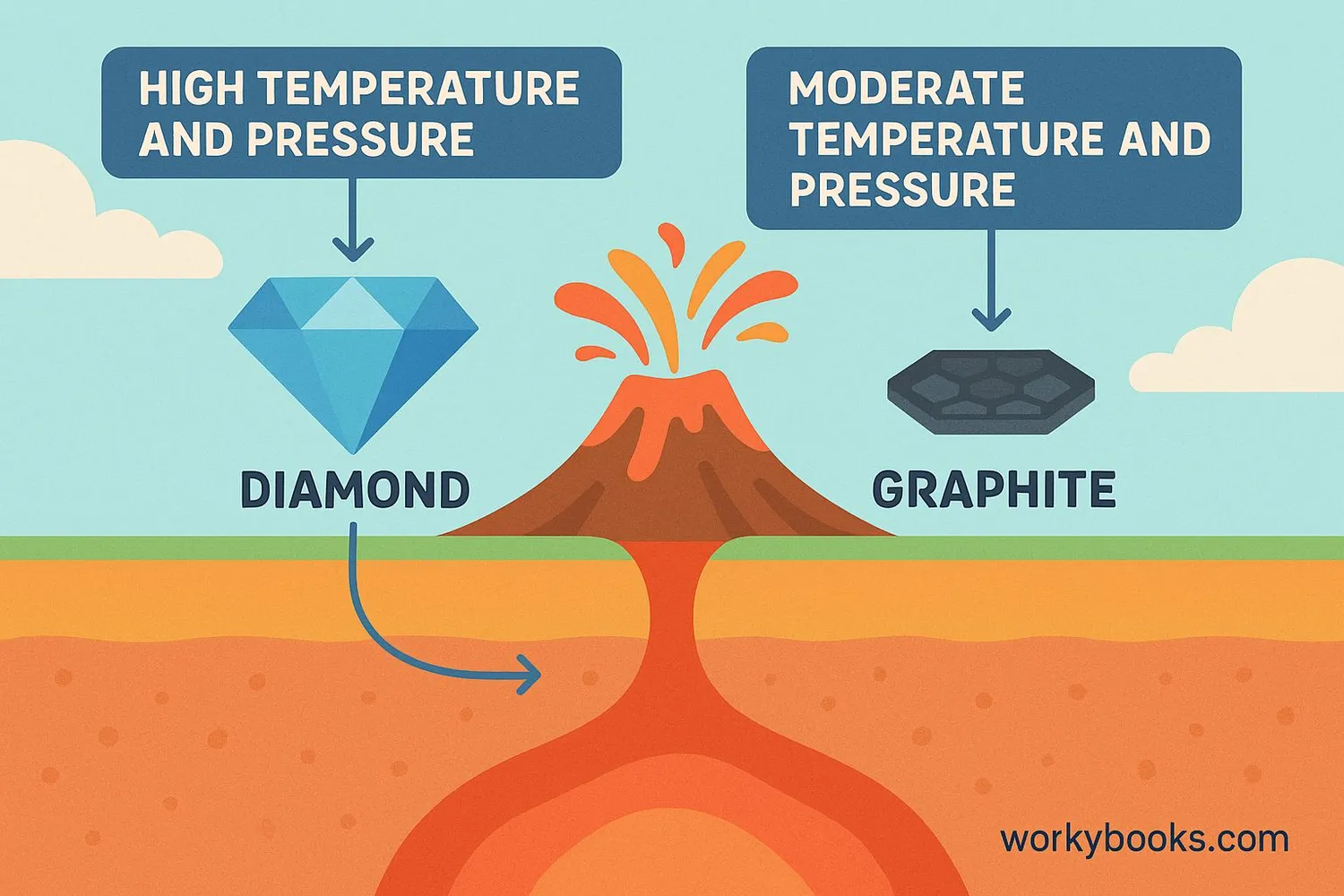
Diamonds form deep underground where there's extreme heat and pressure - it takes billions of years! Volcanic eruptions sometimes bring them to the surface.
Graphite forms at lower temperatures and pressures. It's much more common than diamonds!
Graphene was first made by scientists using special tape to peel layers off graphite. Now they create it in high-tech labs.
Allotropes Quiz
Test your knowledge about allotropes with this fun quiz! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to some common questions about allotropes:
Fun Allotrope Trivia
Discover some amazing facts about allotropes!
Diamond Rain
Scientists believe it rains diamonds on Neptune and Uranus! The extreme pressure in these planets' atmospheres squeezes carbon atoms into diamond crystals that fall like rain.
Pencil Power
A single pencil can draw a line 35 miles long! This is possible because graphite layers easily slide off onto paper - a property that comes from its molecular structure.
Super Material
Graphene is 200 times stronger than steel but incredibly light. A sheet of graphene as thin as plastic wrap could support an elephant without tearing!
Ancient Discovery
People have known about carbon allotropes for thousands of years! Diamonds were prized in ancient India as early as 4th century BC, and graphite was used in pottery in 4th millennium BC.





