Chemical Bonds - Definition, Examples, Quiz, FAQ, Trivia
Discover how atoms connect to form everything around us!
What is a Chemical Bond?
A chemical bond is like a special handshake between atoms! It's the force that holds atoms together to form molecules and compounds. Just like glue holds pieces of paper together, chemical bonds hold atoms together to make everything around us.
Atoms form bonds to become more stable. Most atoms want to have a full outer shell of electrons (like the noble gases). They can achieve this by sharing, giving away, or taking electrons from other atoms.
Science Fact!
Your body contains trillions of chemical bonds that hold your cells together!
Types of Chemical Bonds
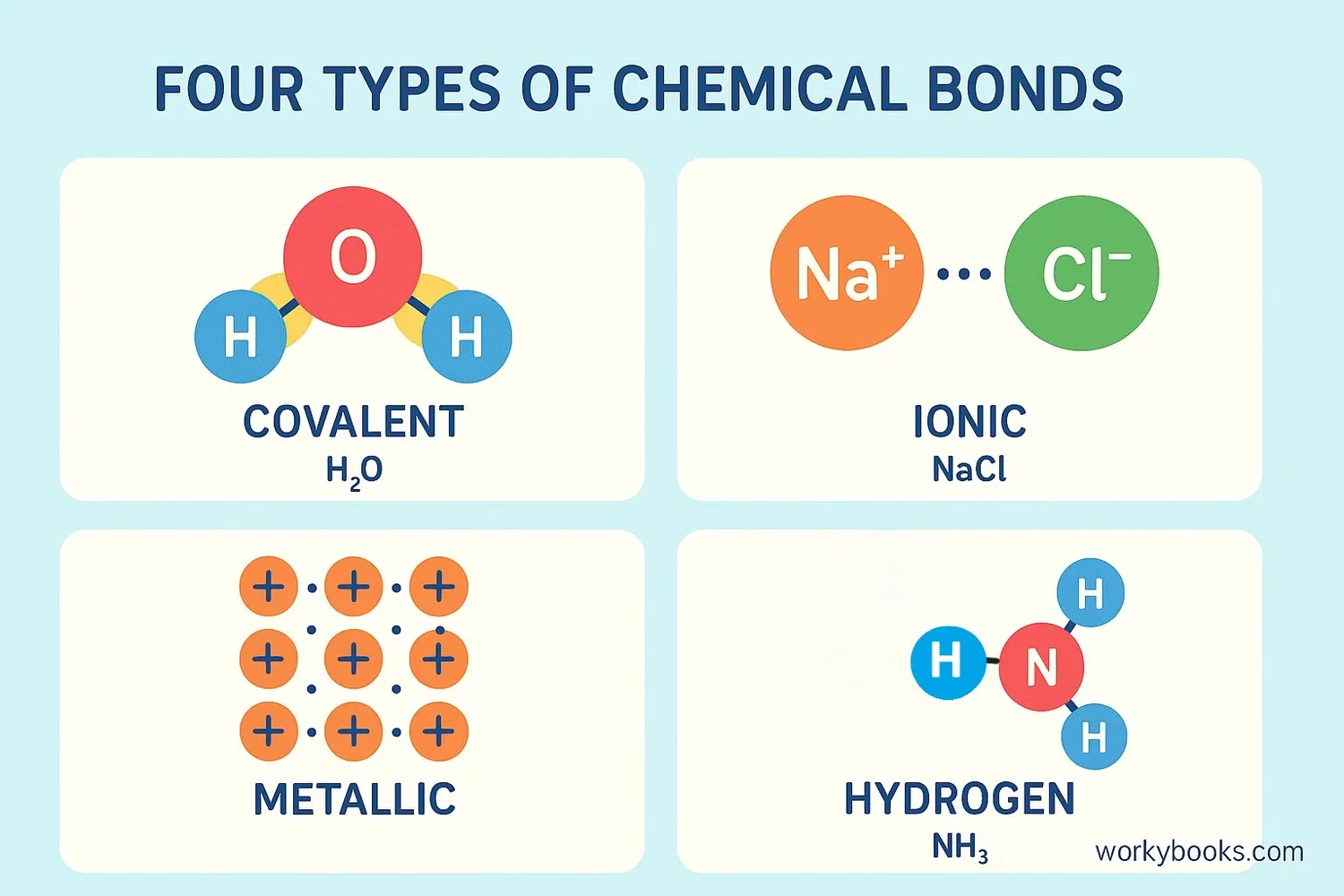
There are four main types of chemical bonds that hold atoms together in different ways:
Covalent Bonds
Atoms share electrons
Example: Water (H₂O) - oxygen shares electrons with two hydrogen atoms
Ionic Bonds
Atoms transfer electrons
Example: Table salt (NaCl) - sodium gives an electron to chlorine
Metallic Bonds
Electrons move freely
Example: Copper wire - electrons flow between metal atoms
Hydrogen Bonds
Weak attraction
Example: Water molecules sticking together
Water's Special Bonds
Water has both covalent bonds (within each molecule) and hydrogen bonds (between molecules). This is why water has such special properties!
Chemical Bond Strength
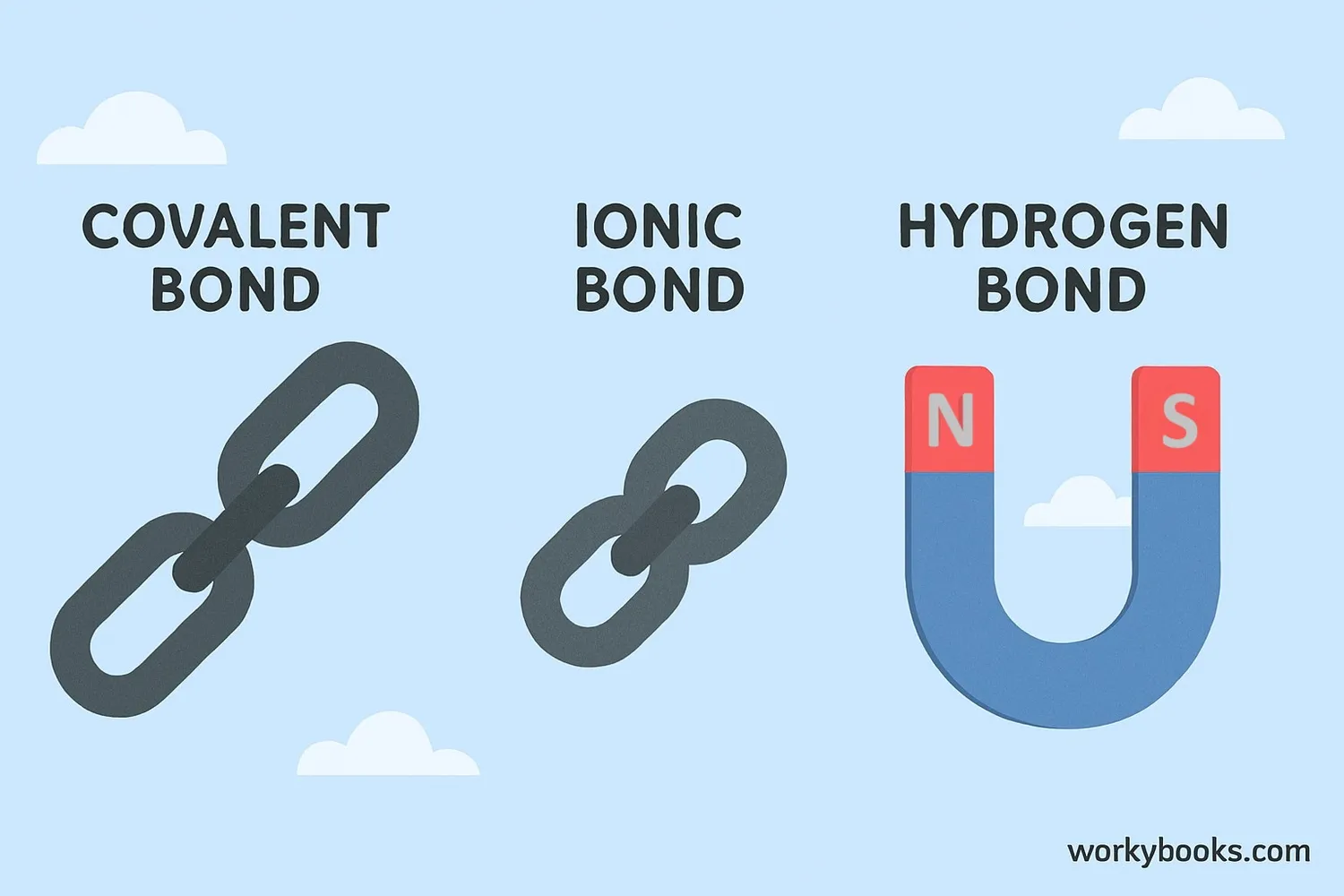
Chemical bonds have different strengths - some are very strong and hard to break, while others are weaker. The strength of a bond determines how much energy is needed to break it:
Covalent Bonds
Strongest bonds
Hard to break
Ionic Bonds
Strong but can dissolve in water
Metallic Bonds
Strong but flexible
Hydrogen Bonds
Weakest bonds
Easy to break
Why does bond strength matter? Strong bonds create stable substances like diamonds (covalent bonds), while weaker bonds like hydrogen bonds allow important biological processes to happen, like DNA unzipping for cell division.
How Chemical Bonds Form
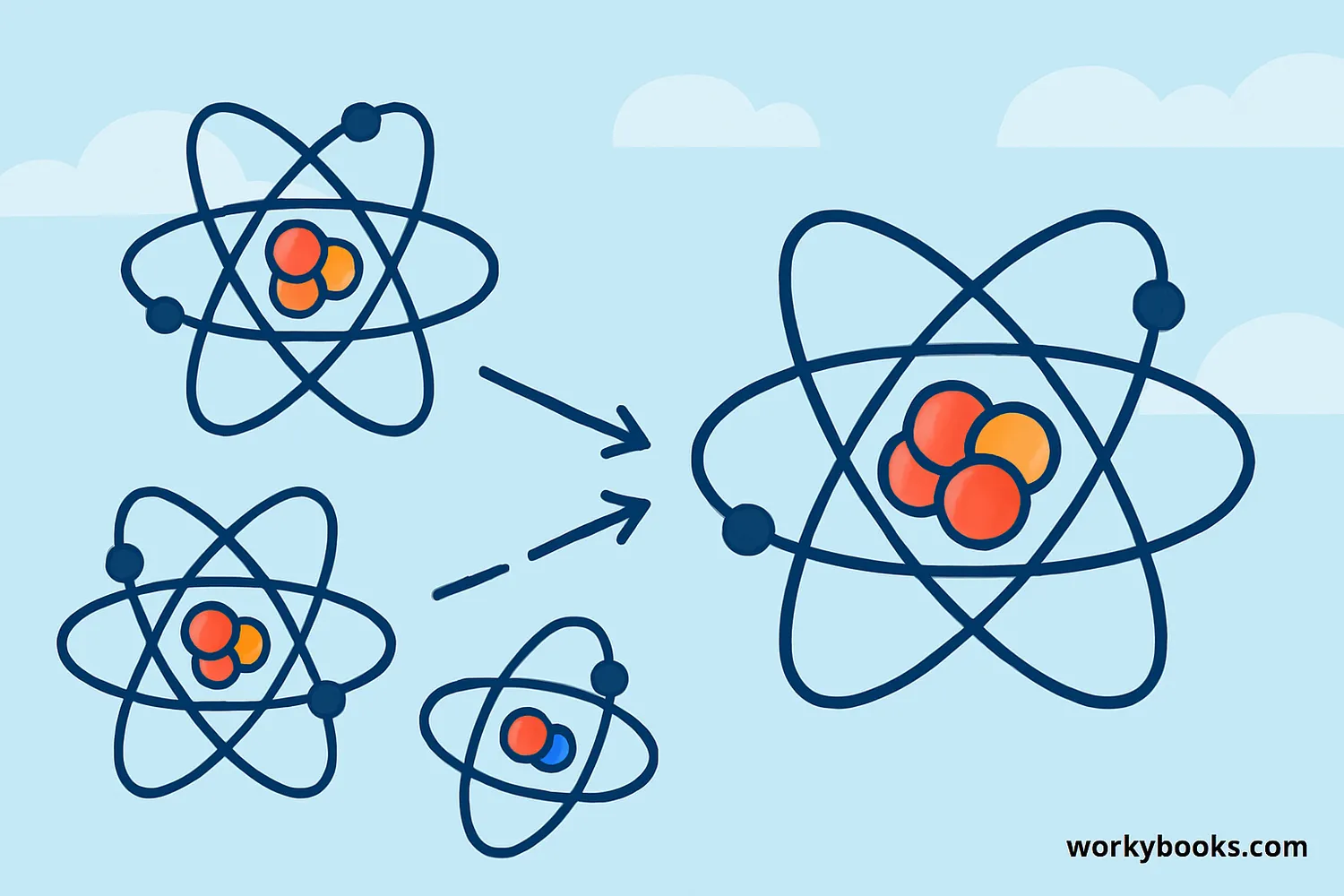
Chemical bonds form when atoms interact with each other. This happens because atoms want to have a full outer shell of electrons. There are three main ways atoms form bonds:
Sharing Electrons
Covalent bonding - atoms share electrons to complete their outer shells
Transferring Electrons
Ionic bonding - one atom gives electrons to another
Pooling Electrons
Metallic bonding - atoms share electrons with many neighbors
When bonds form, energy is usually released. This is why chemical reactions often produce heat or light. Breaking bonds requires energy - that's why you need heat to cook food or melt ice!
Chemical Bonds Quiz
Test your knowledge about chemical bonds! Answer all 5 questions to see how much you've learned.
Frequently Asked Questions
Here are answers to common questions about chemical bonds:
Chemical Bond Trivia
Discover some amazing facts about chemical bonds!
Diamond Strength
Diamonds are the hardest natural substance because of their strong covalent bonds. Each carbon atom is bonded to four other carbon atoms in a rigid structure!
DNA Bonds
Your DNA is held together by hydrogen bonds! These weak bonds allow DNA to "unzip" for copying, which is essential for cell division and life itself!
Space Bonds
In space, chemical bonds form in giant molecular clouds to create new stars and planets. The same bonds that hold water together also form stars!
Conducting Electricity
Metals conduct electricity because of metallic bonds. Their "sea of electrons" can move freely, carrying electrical current through wires.





