Nonpolar Covalent Bonds - Definition, Examples, Quiz, FAQ, Trivia
Discover how atoms share electrons equally to form stable molecules
What are Nonpolar Covalent Bonds?
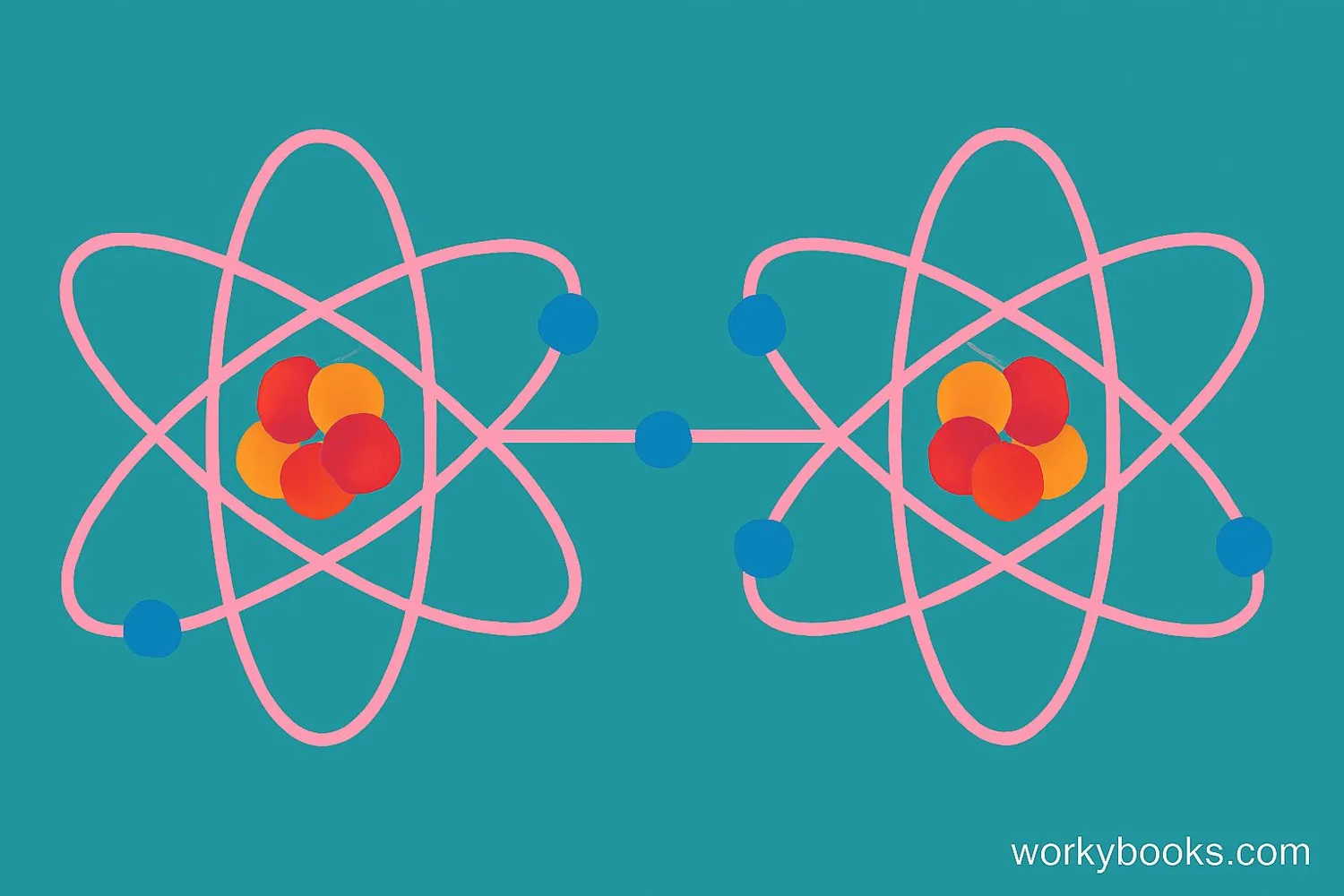
Nonpolar covalent bonds are a type of chemical bond where two atoms share electrons equally. This happens when the atoms have the same or very similar electronegativity - that's a fancy word for how strongly an atom pulls electrons toward itself.
Think of it like two equally strong friends playing tug-of-war with a rope. Neither one is stronger, so the rope stays exactly in the middle. In nonpolar covalent bonds, the electrons spend equal time around both atoms, creating a balanced, stable molecule.
Key Fact!
Nonpolar covalent bonds form between atoms of the same element (like O₂ or H₂) or between atoms with very similar electronegativity values.
How Nonpolar Covalent Bonds Work
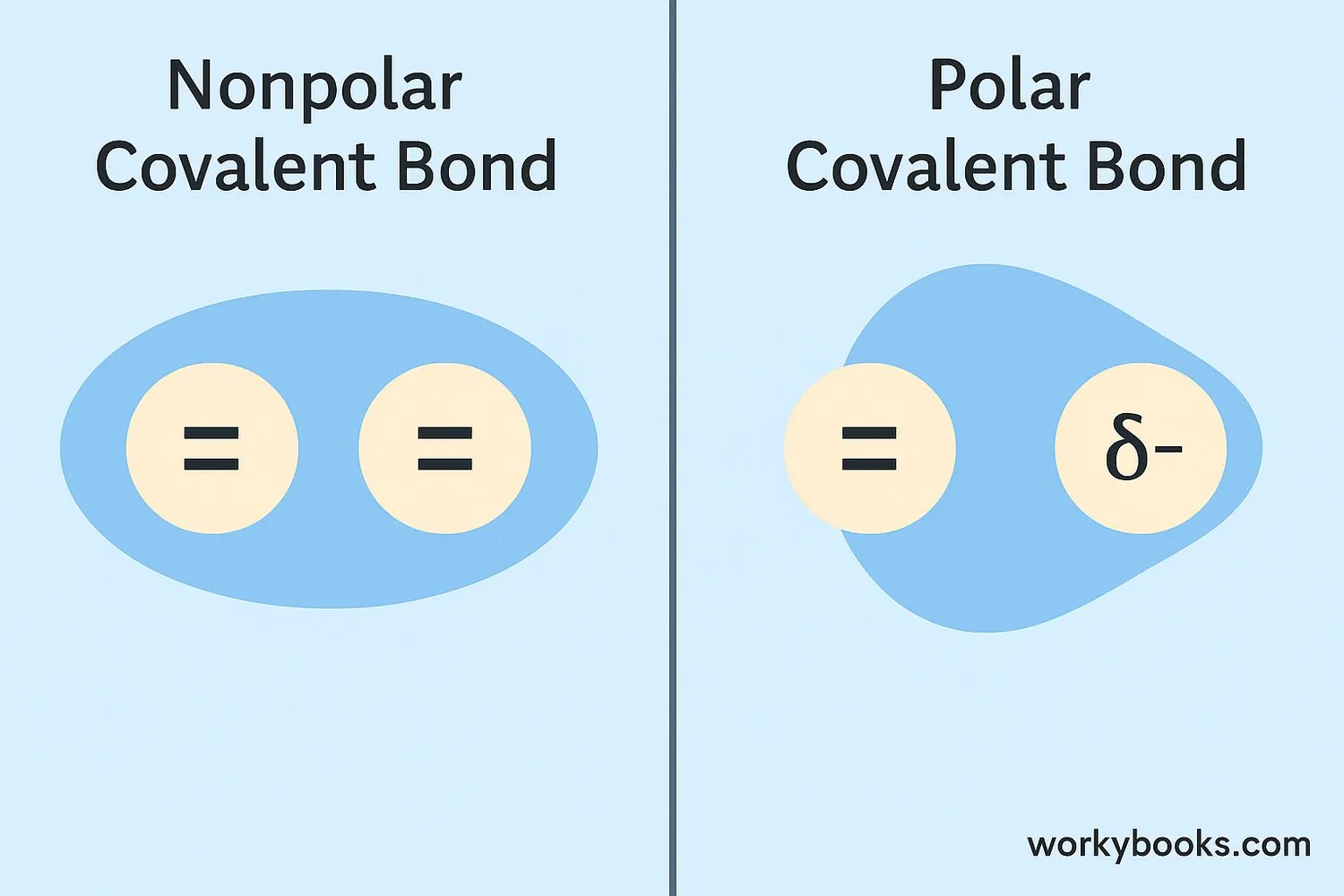
To understand how nonpolar covalent bonds work, we need to know about electronegativity - how much an atom "wants" electrons. When two atoms have the same or very similar electronegativity, they pull on the shared electrons with equal strength.
This creates a bond where the electrons are shared equally, resulting in a balanced molecule with no electrical poles (no positive or negative ends).
Similar Atoms
Two atoms with similar electronegativity approach each other
Electron Sharing
They share one or more pairs of electrons
Equal Pull
Both atoms pull on the electrons with equal strength
Balanced Bond
Electrons are shared equally, creating a nonpolar bond
| Feature | Nonpolar Covalent Bond | Polar Covalent Bond |
|---|---|---|
| Electron Sharing | Equal sharing | Unequal sharing |
| Electronegativity Difference | 0 to 0.4 | 0.5 to 1.7 |
| Charge Distribution | Even | Partial positive and negative ends |
| Example | O₂ (oxygen gas) | H₂O (water) |
Electronegativity Matters!
When the electronegativity difference between two atoms is less than 0.4, the bond is considered nonpolar covalent. From 0.5 to 1.7, it's polar covalent, and above 1.7, it's usually ionic.
Examples of Nonpolar Covalent Bonds
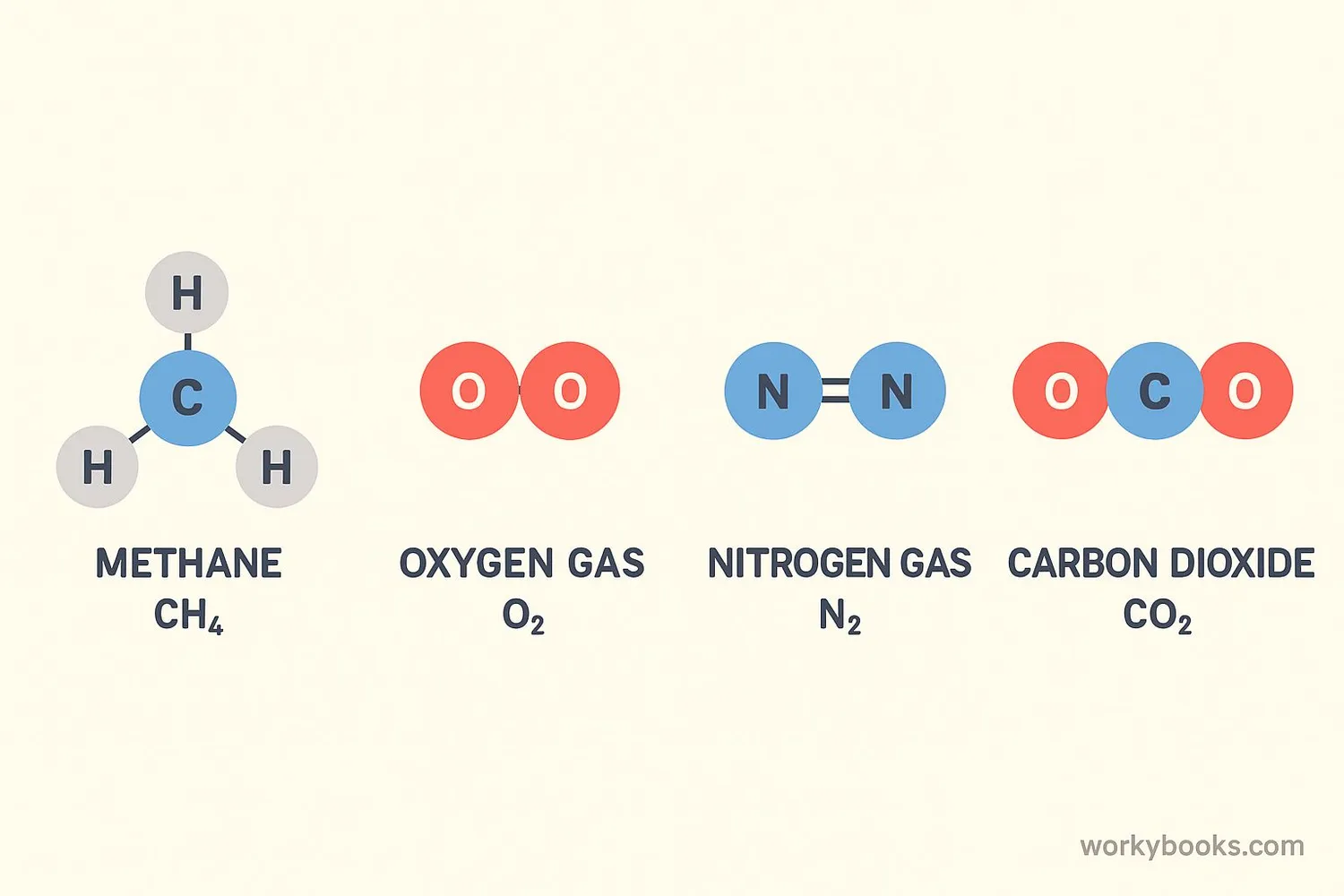
Many common substances around us contain nonpolar covalent bonds. These molecules are often gases at room temperature or liquids that don't mix well with water (like oil). Here are some important examples:
Oxygen Gas (O₂)
Two oxygen atoms with identical electronegativity form a nonpolar double bond
Nitrogen Gas (N₂)
Two nitrogen atoms form a strong nonpolar triple bond
Methane (CH₄)
Carbon and hydrogen have similar electronegativity, forming nonpolar bonds
Other examples include:
• Carbon dioxide (CO₂) - though it has polar bonds, the molecule is linear and symmetrical, making it nonpolar overall
• Hydrogen gas (H₂) - two hydrogen atoms sharing electrons equally
• Chlorine gas (Cl₂) - two chlorine atoms with identical electronegativity
• Many organic compounds like oils and fats
These nonpolar molecules have important properties - they don't dissolve well in water, they often have lower boiling points, and they interact weakly with electric fields.
Nonpolar Covalent Bonds Quiz
Test your knowledge with this quiz! Answer all 5 questions to see how much you've learned about nonpolar covalent bonds.
Frequently Asked Questions
Here are answers to some common questions about nonpolar covalent bonds:
Interesting Facts
Discover some fascinating facts about nonpolar covalent bonds!
Breathing Nonpolar Molecules
The air we breathe is mostly nitrogen (N₂) and oxygen (O₂), both nonpolar molecules! About 78% of air is nitrogen and 21% is oxygen.
Water's Special Properties
Water's polarity (which comes from polar covalent bonds) gives it special properties like high surface tension that allows insects to walk on water!
Carbon's Versatility
Carbon can form nonpolar bonds with itself, creating long chains and rings. This is why there are millions of organic compounds!
DNA's Nonpolar Core
The inside of DNA's double helix has nonpolar bases that stack together. This hydrophobic (water-fearing) core helps stabilize the DNA structure.





